
Ameliorating Effects of Cinnamomum loureiroi and Rosa laevigata Extracts Mixture against Trimethyltin-induced Learning and Memory Impairment Model
© The Korean Society of Medicinal Crop Science. All rights reserved.
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License ( http://creativecommons.org/licenses/by-nc/3.0 ) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
A critical features of Alzheimer’s disease (AD) is cognitive dysfunction, which partly arises from decreased in acetylcholine levels. AD afftected brains are characterized by extensive oxidative stress, which is thought to be primarily induced by the amyloid beta (Aβ) peptide. In a previous study, Cinnamomum loureiroi tincture inhibited acetylcholinesterase (AchE) activity. That study identified AChE inhibitor in the C. loureiroi extract. Furthermore, the C. loureiroi extract enhanced memory in a trimethyltin (TMT)-induced model of cognitive dysfunction, as assessed via two behavioral tests. Rosa laevigata extract protected against oxidative stress-induced cytotoxicity. Administrating R. laevigata extracts to mice significantly reversed Aβ-induced learning and memory impairment, as shown in behavioral tests.
We conducted behavioral to examine the synergistic effects of C. loureiroi and R. laevigata extracts in inhibiting AChE and counteracting TMT-induced learning and memory losses. We also performed biochemical assays. The biochemical results showed a relationship between increased oxidative stress and cholinergic neurons damage in TMT-treated mice.
A diet containing C. loureiroi and R. laevigata extracts ameliorated learning and memory impairments in the Y-maze and passive avoidance tests, and exerted synergistic inhibitory effect against AChE and lipid peroxidation.
Keywords:
Cinnamomum loureiroi, Rosa laevigata, Alzheimer’s Disease, Learning, MemoryINTRODUCTION
Alzheimer’s disease (AD) was originally described in 1906 by Alois Alzheimer based on the observation of amyloid plaques and neurofibrillary tangles during an autopsy of a patient. The primary clinical feature of AD is cognitive dysfunction, which has been demonstrated to be closely related to the loss of the cholinergic function (Berchtold et al., 1998).
It has been frequently reported that AD patients display a decrease in acetylcholine (ACh) levels within the basal forebrain (McGleenon et al., 1999). ACh is an important neurotransmitter that plays a critical role in learning and memory processes. In the central cholinergic systems, ACh is synthesized from choline and acetyl-coenzyme A by choline acetyltransferase (ChAT) (Ohno et al., 2001). ChAT and acetylcholinesterase (AChE) are responsible for the synthesis and hydrolysis of ACh in cholinergic neurons, respectively. AChE inhibitors that inhibit the hydrolysis of ACh to increase the levels of cholinergic neurotransmitters are widely administered to patients with AD. Synthetic AChE inhibitors, including tacrine, donepezil, and rivastigmine, have been tested clinically. However, the side effects of these drugs have led to the development of new AChE inhibitors from nontoxic natural resources.
Cinnamomum loureiroi contains large amounts of bioactive molecules, including essential oils, tannin, mucus, and coumarins. C. loureiroi is one of the most widely used herbal medicines in Korea, belongs to the family Lauraceae, and is often used as a spice and flavoring agent.
Trimethyltin (TMT) is a compound that induces neuronal degeneration in the hippocampus, and results in behavioral alternations including cognitive impairments. TMT has been widely used as a neurotoxin because of its effects associated with in vivo behavioral changes and biochemicaldeficits in the brains (Choi et al., 2016).
Our study have previously demonstrated that the C. loureiroi extract significantly inhibited AChE in vitro and was neuroprotective against TMT-induced cognitive dysfunction in mice (Kim et al., 2016). We further identified 2,4-bis-(1,1- dimethylethyl) phenol from C. loureiroi as a potential AChE inhibitor.
AD brains are characterized by extensive oxidative stress, which is believed to be primarily induced by the amyloid beta (Aβ) peptide. Many investigators surmise that deposition of Aβ peptide is the crucial step in AD pathogenesis. Aβ overproduction leads to the intracellular accumulation of reactive oxygen species (ROS), which eventually results in the peroxidation of the cells, modification of proteins, DNA/RNA damage, and cell death (Butterfield et al., 2007).
Rosa laevigata, a plant of the Rosaceae family, is native to China and Japan. It tastes sour and sweet and passes to kidney, urinary bladder, and large intestine channels. R. laevigata relieves nocturnal emission and leucorrhagia and reduces the frequency of urination.
We have previously investigated the protective effects of R. laevigata aginst Aβ-induced oxidative stress and learning and memory impairment in mice. The R. laevigata extract exerted an anti-amnestic activity in vivo through the blockade of Aβ-induced neuronal damage. Our previous findings also indicated that the active component of R. laevigata is the 1,2-benzenedicarboxylic acid, dinonyl ester (Choi et al., 2006).
AChE is a potent amyloid-promoting factor when compared with other Aβ-associated proteins. Thus, in addition to its role in the cholinergic synapses, AChE may function by accelerating Aβ formation and could play a role during amyloid deposition in AD. Previous studies suggested that Aβ injection directly inhibits various cholinergic neuronal functions. These previous results specifically indicated the potential role of free radical toxicity and the damage of cholinergic neurons, demonstrating an increase in oxidative stress and AChE activity (Nabeshima and Nitta, 1994; Choi et al., 2009). These results indicated an interaction between AChE and Aβ.
In this present study, to test the potential effects of the combination of the AChE inhibitory effect of C. loureiroi and the anti-oxidative effect of R. laevigata. The possible protective effects of C. loureiroi and R. laevigata extracts against TMT-induced neuronal damage were examined.
To investigate the effect of the extracts on learning and memory impairment in mice, in vivo behavioral and biochemical assays were performed. The present biochemical results demonstrated a relationship between increased oxidative stress and damaged cholinergic neurons in TMT-treated mice. The damage induced by TMT injection was potently reversed by the C. loureiroi and R. laevigata extracts.
MATERIALS AND METHODS
1. Materials
Acetylthiocholine iodide, 5,5ʹ-dithiobis-(2-nitro)benzoic acid (DTNB), dimethyl sulfoxide (DMSO), and TMT were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were of analytical grade unless otherwise specified.
2. Sample preparation
Dried Cinnamomum loureiroi and Rosa laevigata were purchased from Gyung-dong market, an oriental medicine store in Seoul, South Korea. The plant materials were authenticated by the Institute of Biotechnology at Korea University.
The dried C. loureiroi and R. laevigata were ground into a fine powder form and subsequently extracted with 10 volumes of 70% ethanol. The samples were placed on a shaker over 12 h at 1.57 × g. The extract was filtered through a No. 42 filter paper (Whatman Co., Maidstone, England). The resulting ethanol extracts were evaporated using a rotary evaporator (Eyela, Tokyo Rikakikai Co., Ltd., Tokyo, Japan) under reduced pressure at 37℃ (Choi et al., 2016).
3. Cell culture
The PC12 cell line (CRL-1721) was obtained from the American Type Culture Collection (Manassas, VA, USA). The PC12 cells were cultured in RPMI 1640 medium supplemented with 10% (v/v) horse serum from a donor herd, 5% (v/v) fetal bovine serum, and 1% (v/v) antibiotic-antimycotic (Gibco BRL, Gaithersburg, MD, USA). The cells were cultured on 100㎜ diameter tissue culture dishes (BD Biosciences, Franklin Lakes, NJ, USA) and maintained in an incubator (37℃, water saturated, and 5% CO2 atmosphere). The cells were sub-cultured when a confluency of 80 - 90% (split ratio 1 : 4) was attained. The medium was refreshed approximately three times a week (Choi et al., 2013).
4. Measurement of AChE activity
The AChE activity was determined using the modified spectrophotometric method of Ellman. ACh iodide was used as the reaction substrate, and DTNB was used to measure AChE activity (Ellman et al., 1961; Kim et al., 2011).
Briefly, for the enzyme preparation, PC12 cells were homogenized using a homogenizer (Glas-Col, Terre Haute, IN, USA) with Tris-hydrochloride (HCl) buffer (20 mM Tris-HCl (pH 7.5) containing 150 mM sodium chloride (NaCl), 10 mM magnesium chloride (MgCl), and 0.5% Triton X-100). After homogenization, the samples were centrifuged at 10,000 × g for 15 min and the resulting supernatants were used as the enzyme source.
The protein concentration was determined using a protein quantification assay kit (Bio-Rad, Hercules, CA, USA), with bovine serum albumin as the protein standard. 10㎕ of each sample were mixed with 10㎕ of enzyme solution, and were subsequently added to 70㎕ of the reaction mixture [50 mM sodium phosphate buffer (pH 8.0) containing 0.5 mM ACh iodide and 1 mM DTNB], and incubated at 37℃ for 15 min. The ACh iodide and enzyme reaction were monitored at a wavelength of 405㎚ using a 96-well micro plate reader (GENios, Tecan Ltd., Männedorf, Switzerland).
5. Animals
Institute of Cancer Research (ICR) mice (5-week-old males) were purchased from DBL (Eumseong, Korea) and were provided access to food and water ad libitum. The C. loureiroi and R. laevigata extracts were mixed into the feed. Subsequently, TMT, which was dissolved in an NaCl solution, was administered through intraperitoneal (IP) injection. The control group was injected with NaCl without TMT.
All sample groups were injected with TMT (100㎕ per mouse; 2.5㎎/㎏ body weight) (Choi et al., 2016). However, the animals of each group were maintained on diets differentially supplemented with C. loureiroi and R. laevigata extracts (30 or 50㎎/㎏ per day, respectively) for 4 weeks. All experimental procedures abided by the guidelines established by the Animal Care and Use Committee of Korea University (KUIACUC-2017-142).
6. Y-maze test
The Y-maze test was performed 2 days after the TMT injection. The maze consisted of black plastic, with three maze arms measuring each 33㎝ long, 15㎝ high, 10㎝ wide, and separated by an angle of 120o. Each mouse was placed at the end of one arm and allowed to move freely through the maze during an 8-min period. The sequence of arm entries was recorded manually.
Spontaneous alternation behavior was defined as entry into all three arms by consecutive choice in overlapping triplet sets. The percentage of spontaneous alternation behavior was calculated as the ratio of actual-to-possible alternations (Kim et al., 2017).
7. Passive avoidance test
The passive avoidance test was performed 2 days after the TMT injection. The apparatus consisted of an illuminated chamber connected to a dark chamber.
An acquisition trial was performed on day 1. Each mouse was placed in the apparatus and was left there for 1 min with no light or shock, followed by a 2 min period with light and no shock, to habituate to the apparatus. Subsequently, the mice were individually placed in the illuminated chamber. Immediately after entering the dark chamber, an inescapable scrambled electric shock (0.5㎃ for 1 s) was delivered through the floor grid (acquisition trial). The mice were subsequently returned to their cages. Each mouse was placed again in the illuminated chamber 24 h later (retention trial).
The interval between placement in the illuminated chamber and entry into the dark chamber was measured as the latency. The maximum testing limit for step-through latency was 300 s (Kim et al., 2017).
8. Quantification of ACh content in mice brain tissues
The ACh brain content was measured using the method of Hestrin as described previously based on the reaction of ACh and hydroxylamine (Hestrin, 1949).
Mice were sacrificed, and their brains were dissected and maintained at −80℃ prior to use. The brains were homogenized in cold phosphate buffered saline (PBS) in an ice bath. The homogenates were directly centrifuged twice at a 30 s interval at 33,600 × g for 10 s. Subsequently, 1㎖ of the homogenate was mixed with 2㎖ alkaline hydroxylamine reagent. After at least 1 min, the pH was adjusted to 1.2 ± 0.2 with 1㎖ HCl solution and 1㎖ iron solution. The density of the purple brown color was determined at 540㎚.
9. Determination of lipid peroxidation in mice brain tissues
The brains were homogenized in cold PBS in an ice bath. The homogenates were directly centrifuged twice at a 30 s interval at 33,600 × g for 10 s. Aliquots of the supernatant were used to determine the malondialdehyde (MDA) levels and protein content in the brain.
The MDA level was assayed for lipid peroxidation products by using previously method (Choi et al., 2009). Briefly, 80㎕ of each homogenate were mixed with 480㎕ phosphoric acid (1%, v/v) followed by the addition of 160㎕ thiobarbituric acid solution (0.67%, w/v). The mixture was incubated at 95℃ in a water bath for 45 min. After cooling, the colored complex was extracted with n-butanol. The butanol phase was separated by centrifugation, and the absorbance was measured using tetramethoxypropane as a standard at 532㎚.
10. Statistical analysis
All data were expressed as means ± standard deviation (SD). The data were analyzed using Duncan’s Multiple Range Tests (DMRT) in statistical analysis system (SAS Institute Inc., Cary, NC, USA). The statistical significance of differences among groups was calculated by One-way (ANOVA). The statistical difference was set at p < 0.01.
RESULTS AND DISCUSSION
The cholinergic system impairment observed in patients with AD leads to the cognitive and behavioral dysfunctions commonly associated with dementia. The cholinergic deficit in patients with AD is demonstrated by reduced ChAT activity and increased AChE activity. Based on these previous findings, there has been increasing interest in developing AChE inhibitors for therapeutic use in AD. AChE inhibitors decrease the hydrolysis of ACh in the brain, thereby boosting cholinergic neurotransmission. This effect was based on the premise that boosting ACh levels would attenuate the neuronal deficits and cognitive impairments present in patients with AD (McGleenon et al., 1999).
Aβ is known to increase oxidative stress in nerve cells. This oxidative stress is mediated by ROS, which are generated continuously in the nervous tissue during normal metabolism and neuronal activity. When the metabolic balance between ROS and the antioxidant system is lost, oxidative stress occurs. Free radicals, including hydrogen peroxide, are generated by multiple enzymatic and nonenzymatic pathways, leading to the initiation of the oxidative stress cascade (Smith et al., 2007; Jung et al., 2012; Ko and Lee, 2015).
Previous reports indicated that AChE accelerates amyloid formation, and is a potent amyloid-promoting factor when compared with other Aβ-associated proteins. Thus, AChE may function by accelerating Aβ formation and could play a role during amyloid deposition in AD (Inestrosa et al., 1996). In our previous study, we demonstrated that Cinnamomum loureiroi extract significantly inhibited AChE in vitro and was neuroprotective against TMT-induced cognitive dysfunction in mice. The Rosa laevigata extract showed anti-amnestic activity in vivo through the blockade of Aβ-induced neuronal damage. In this study, the possible synergistic protective effect of C. loureiroi and R. laevigata extracts against TMT-induced neuronal damage was examined.
In order to confirm the synergistic effect of C. loureiroi and R. laevigata extracts, the AChE inhibitory effect was evaluated using Ellman’s method. As shown in Fig. 1, mixtures of various ratios of the extracts were tested and the 70 : 30 ratio resulted in the highest effect (22%). Our study previously reported that the extract of C. loureiroi significantly inhibited AChE, and identified 2,4-bis(1,1- dimethylethyl)phenol from C. loureiroi as a potential AChE inhibitor. Also 1,2-benzenedicarboxylic acid dinonyl ester from R. laevigata demonstrated AChE inhibitory effect. It is believed that the AChE inhibitory effect has been shown in these components.
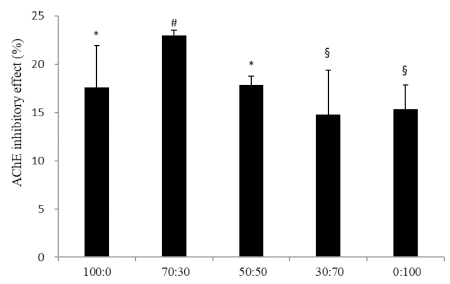
Acetylcholinesterase (AChE) inhibition by C. loureiroi and R. laevigata extracts.The sample groups were treated with various mixtures of C. loureiroi and R. laevigata extracts (100 : 0, 70 : 30, 50 : 50, 30 : 70, 0 : 100, w/w). The concentration of all samples was 1 ㎎/㎖. Each value represents the means ± SD (n = 4), p < 0.01. Different superscript symbols (*, §, and #) represent
This study demonstrated that C. loureiroi and R. laevigata extracts inhibited AChE in vitro, and that the 70 : 30 mixture resulted in a improved effect when compared with other mixtures. In vivo experiment, compared 50 : 50 and 70 : 30 which showed the best effect in vitro experiment. To determine the anti-amnestic effects of C. loureiroi and R. laevigata extracts combination in vivo, a Y-maze test was performed using TMT-treated mice.
TMT is considered a useful tool to induce neurodegeneration in vivo. Animals exposed to TMT develop behavioral alterations featuring cognitive impairments. Furthermore, TMT induces selective neuronal cell death (Geloso et al., 2011).
Consistently with previous reports, our study confirmed that exposure to TMT impaired learning and memory in mice. The Y-maze test was performed 3 days after the TMT injection, and resulted in significant impairments of the spatial working memory (34% decrease in the Y-maze alternation behavior) in TMT-treated mice compared with the control group. However, the TMT-induced decrease in working memory was reversed to the control level by pretreatment with the 70 : 30 extract mixture, namely 30㎎/㎏ and 50㎎/㎏ of C. loureiroi and R. laevigata extracts, respectively, per day (Fig. 2).
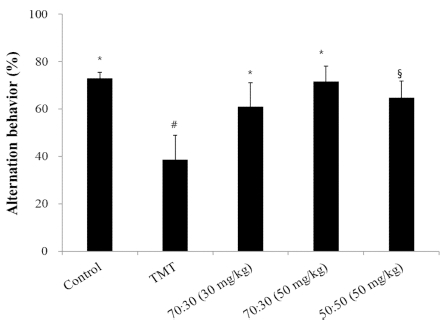
The effect of C. loureiroi and R. laevigata extracts on TMT-induced memory deficit in mice as measured by a Y-maze test.Spontaneous alternation behavior was measured over 8 min. The control group was injected with a sodium chloride solution, while the TMT group was injected with a sodium chloride solution containing TMT. Sample groups were injected with TMT solution after pretreatment with C. loureiroi and R. laevigata extracts (70 : 30, 30, or 50㎎/㎏ of body weight per day; 50 : 50, 50㎎/㎏ of body weight per day, respectively). Each value represents the means ± SD (n = 8), p < 0.01. Different superscript symbols (*, §, and #) represent statistical differences between groups.
This improvement was similar to the 400 ㎎/㎏ per day concentration of C. loureiroi ethanol extract in our previous study (Kim et al., 2016). The number of arm entries did not change among any of the experimental groups (data not shown), indicating an intact general locomotion following the TMT injection or the sample diet. These results suggested that the behavioral changes observed were specific to the extract-associated attenuation in spatial memory.
The passive avoidance test was performed 3 days after the TMT injection, and resulted in a significant reduction (80% decrease) in step-through latency in the TMT-treated mice when compared with the control group. Administering a diet consisting of the C. loureiroi and R. laevigata extracts attenuated the TMT-induced impairment in the passive avoidance test (Fig. 3).
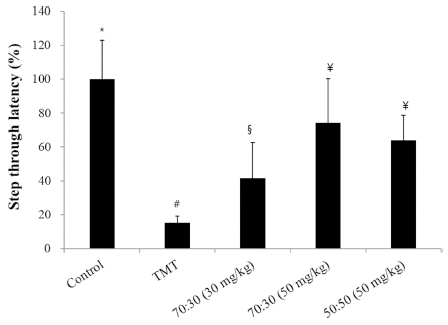
The effect of the C. loureiroi and R. laevigata extracts on TMT-induced memory deficits in mice as assessed with a passive avoidance test.Step-through latency was measured over 5 min. The control group was injected with a sodium chloride solution. The TMT group was injected with a sodium chloride solution containing TMT. Sample groups were injected with TMT solution after pretreatment with C. loureiroi and R. laevigata (70 : 30, 30, or 50㎎/㎏ of body weight per day; 50 : 50, 50 ㎎/㎏ of body weight per day, respectively). Each value represents the means±SD (n=8), p<0.01. Different superscript symbols (*, #, §, and ¥) represent statistical differences between groups.
Our findings suggested that a diet containing the extracts of C. loureiroi and R. laevigata had a protective effect against learning and memory deficits induced by TMT. Furthermore, the 70 : 30 mixture indicated a dosedependent attenuation of TMT-induced impairment in the Y-maze and passive avoidance test. Thus, the extract displayed a significant anti-amnestic effect in our mouse model.
Based on these results, it is possible that the AChE inhibitory effects of the extracts combination may account for the anti-amnestic effects observed in this study. The 70 : 30 concentration showed a statistically significant increase in vitro experiments. Although the activity effect did not seem to be much, it showed a significant effect even at low concentrations in Y-maze test. Following the behavioral tests, the mice serum was collected to evaluate the acute toxicity of C. loureiroi and R. laevigata extracts mixtures using the serum transaminase reagents kit. Serum aminotransferases were not significantly different among the experimental groups, indicating a lack of liver toxicity (data not shown).
The ACh content in the brains of the experimental groups was determined by spectrophotometric analysis. As shown in Fig. 4, ACh was significantly decreased in the TMT group (about 20%). However, the ACh levels were increased to the level of the controls following treatment with C. loureiroi and R. laevigata extracts. The sample groups which were administered the 70 : 30 (30㎎/㎏, 50㎎/㎏) and 50 : 50 (50㎎/㎏) diet mixtures recovered almost to the same level as the control group.
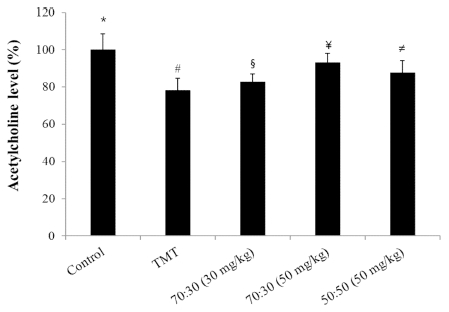
Effect of C. loureiroi and R. laevigata extracts on acetylcholine content in mice brains treated with TMT.The control group was injected with a sodium chloride solution. The TMT group was injected with a sodium chloride solution containing TMT. Sample groups were injected with TMT solution after pretreatment with C. loureiroi and R. laevigata extracts (70 : 30, 30㎎/㎏ or 50㎎/㎏ of body weight per day; 50 : 50, 50㎎/㎏ of body weight per day, respectively). Each value represents the means ± SD (n=8). p<0.01. Different superscript symbols (*, #, §, ¥, and ≠) represent statistical differences between groups.
One of the mechanisms of TMT-induced neurodegeneration is the increase in AChE activity. These changes were correlated with the degeneration of cholinergic axons and changes in ACh levels and their receptors. Indeed, TMT toxicity has been reported to cause a decrease of ACh release in cerebral tissues (Loullis et al., 1985).
As shown in Fig. 2 and 3, the administration of C. loureiroi and R. laevigata extracts in the diet of TMT treated mice attenuated TMT-induced learning and memory impairments. The group administered C. loureiroi and R. laevigata extracts exhibited increased ACh levels compared with the levels in the TMT alone group. Thus, C. loureiroi and R. laevigata extracts possibly improved the learning and memory ability in TMT-treated mice by inhibiting AChE and consequently restoring ACh levels.
Another possible mechanism is that TMT-induced neuronal damage generates ROS. Previous reports demonstrated that TMT promotes the formation of a major product of lipid peroxidation (Wang et al., 2008; Shuto et al., 2009). To determine the TMT-induced lipid peroxidation in the mice brains, MDA was measured after the behavioral tests. The MDA levels increased significantly (1.16 μM/㎎ protein) in the TMT-injected group when compared with those of the control group. The increase in MDA levels indicated an elevation of lipid peroxidation in the brains of TMTinjected mice. Diets containing C. loureiroi and R. laevigata extracts reversed the effects of TMT-induced lipid peroxidation (Fig. 5). Although 70 : 30 (30㎎/㎏, 50㎎/㎏) are not statistically different from 50 : 50 (50㎎/㎏), it is a meaningful result to show its effect even at low concentrations (30㎎/㎏).
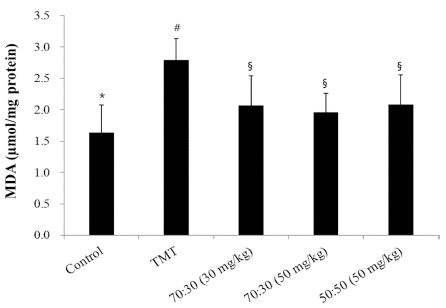
The effect of C. loureiroi and R. laevigata extracts on lipid peroxidation in mice brains.The control group was injected with a sodium chloride solution. The TMT)group was injected with a sodium chloride solution containing TMT. Sample groups were injected with TMT solution after pretreatment with C. loureiroi and R. laevigata extracts (70 : 30, 30, or 50㎎/㎏ of body weight per day; 50 : 50, 50㎎/㎏ of body weight per day). Each value represents the means ± SD (n = 8). p < 0.01. Different superscript symbols (*, #, and §) represent statistical differences between groups.
These findings indicated that these changes may be the mechanisms underlying the improvement of learning and memory ability in mice by attenuating AChE and oxidative stress. The pretreatment with the C. loureiroi and R. laevigata extracts mixture reversed the ACh reduction and MDA elevation in mice treated with TMT. Taken together, our present findings indicated that C. loureiroiand R. laevigata extracts mixture inhibited TMT-induced neuronal cell damage and improved TMT-induced learning and memory deficits.
ACKNOWLEDGMENTS
This research was supported by High Value-Added Food Technology Development Program, Ministry of Agriculture, Food and Rural Affairs, Republic of Korea.
References
- N.C. Berchtold, C.W. Cotman, Evolution in the conceptualization of dementia and Alzheimer ?(tm)s disease: Grecoroman period to the 1960s., Neurobiol. Aging, (1998), 19, p173-189.
- D.A. Butterfield, T. Reed, S.F. Newman, R. Sultana, Roles of amyloid I -peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer ?(tm)s disease and mild cognitive impairment., Free Radic. Biol. Med, (2007), 43, p658-677.
-
S.J. Choi, J.K. Kim, H.K. Kim, K. Harris, C.J. Kim, G.G. Park, C.S. Park, D.H. Shin, 2,4-Di-tert-butylphenol from sweet potato protects against oxidative stress in PC12 cells and in mice., J. Med. Food, (2013), 16, p977-983.
[https://doi.org/10.1089/jmf.2012.2739]

-
S.J. Choi, M.J. Kim, H.J. Heo, H.K. Kim, B. Hong, C.J. Kim, B.G. Kim, D.H. Shin, Protective effect of Rosa laevigata against amyloid beta peptide-induced oxidative stress., Amyloid, (2006), 13, p6-12.
[https://doi.org/10.1080/13506120500535636]

- S.J. Choi, M.J. Kim, H.J. Heo, J.K. Kim, W.J. Jun, H.K. Kim, E.K. Kim, M.O. Kim, H.Y. Cho, H.J. Hwang, Y.J. Kim, D.H. Shin, Ameliorative effect of 1,2-benzenedicarboxylic acid dinonyl ester against amyloid beta peptide-induced neurotoxicity., Amyloid, (2009), 16, p15-24.
-
S.J. Choi, S.S. Oh, C.R. Kim, Y.K. Kwon, S.H. Suh, J.K. Kim, G.G. Park, S.Y. Son, D.H. Shin, Perilla frutescens extract ameliorates acetylcholinesterase and trimethyltin chlorideinduced neurotoxicity., J. Med. Food, (2016), 19, p281-289.
[https://doi.org/10.1089/jmf.2015.3540]

-
G.L. Ellman, K.D. Courtney, V. Andres Jr, R.M. Featherstone, A new and rapid colorimetric determination of acetylcholinesterase activity., Biochem. Pharmacol, (1961), 7, p88-95.
[https://doi.org/10.1016/0006-2952(61)90145-9]

-
M.C. Geloso, V. Corvino, F. Michetti, Trimethyltininduced hippocampal degeneration as a tool to investigate neurodegenerative processes., Neurochem. Int, (2011), 58, p729-738.
[https://doi.org/10.1016/j.neuint.2011.03.009]

- S. Hestrin, The reaction of acetylcholine and other carboxylic acid derivatives with hydroxylamine, and its analytical application., J. Biol. Chem, (1949), 180, p249-261.
-
N.C. Inestrosa, A. Alvarez, C.A. Pérez, R.D. Moreno, M. Vicente, C. Linker, O.I. Casanueva, C. Soto, J. Garrido, Acetylcholinesterase accelerates assembly of amyloid-β-peptides into Alzheimer's fibrils: Possible role of the peripheral site of the enzyme., Neuron, (1996), 16, p881-891.
[https://doi.org/10.1016/s0896-6273(00)80108-7]

-
M.H. Jung, K.S. Song, Y.H. Seong, Inhibitory effect of Chaenomeles sinensis fruit on amyloid β protein (25-35)-induced neurotoxicity in cultured neurons and memory impairment in mice., Hanguk Yakyong Changmul Hakhoe Chi, (2012), 20, p8-15.
[https://doi.org/10.7783/kjmcs.2012.20.1.008]

- C.R. Kim, S.J. Choi, J.K. Kim, C.K. Park, M.C. Gim, Y.J. Kim, G.G. Park, D.H. Shin, 2,4-Bis(1,1-dimethylethyl)phenol from Cinnamomum loureirii improves cognitive deficit, cholinergic dysfunction, and oxidative damage in tmt-treated mice., Biol. Pharm. Bull, (2017), 40, p932-935.
- C.R. Kim, S.J. Choi, Y.K. Kwon, J.K. Kim, Y.J. Kim, G.G. Park, D.H. Shin, Cinnamomum loureirii extract inhibits acetylcholinesterase activity and ameliorates trimethyltin-induced cognitive dysfunction in mice., Biol. Pharm. Bull, (2016), 39, p1130-1136.
-
J.K. Kim, S.J. Choi, H. Bae, C.R. Kim, H.Y. Cho, Y.J. Kim, S.T. Lim, C.J. Kim, H.K. Kim, S. Peterson, D.H. Shin, Effects of methoxsalen from poncirus trifoliata on acetylcholinesterase and trimethyltin-induced learning and memory impairment., Biosci. Biotechnol. Biochem, (2011), 75, p1984-1989.
[https://doi.org/10.1271/bbb.110386]

-
C.C. Loullis, R.L. Dean, A.S. Lippa, D.E. Clody, J. Coupet, Hippocampal muscarinic receptor loss followingtrimethyl tin administration., Pharmacol. Biochem. Behav, (1985), 22, p147-151.
[https://doi.org/10.1016/0091-3057(85)90498-8]

- B.M. McGleenon, K.B. Dynan, A.P. Passmore, Acetylcholinesterase inhibitors in Alzheimer’s disease., Br. J. Clin. Pharmacol, (1999), 48, p471-480.
- T. Nabeshima, A. Nitta, Memory impairment and neuronal dysfunction induced by Iβ-amyloid protein in rats., Tohoku J. Exp. Med, (1994), 174, p241-249.
-
K. Ohno, A. Tsujino, J.M. Brengman, C.M. Harper, Z. Bajzer, B. Udd, R. Beyring, S. Robb, F.J. Kirkham, A.G. Engel, Choline acetyltransferase mutations cause myasthenic syndrome associated with episodic apnea in humans., Proc. Natl. Acad. Sci. USA, (2001), 98, p2017-2022.
[https://doi.org/10.1073/pnas.98.4.2017]

-
M. Shuto, K. Higuchi, C. Sugiyama, M. Yoneyama, N. Kuramoto, R. Nagashima, K. Kawada, K. Ogita, Endogenous and exogenous glucocorticoids prevent trimethyltin from causing neuronal degeneration of the mouse brain in vivo: Involvement of oxidative stress pathways., J. Pharmacol. Sci, (2009), 110, p424-436.
[https://doi.org/10.1254/jphs.09107fp]

- D.G. Smith, R. Cappai, K.J. Barnham, The redox chemistry of the Alzheimer’s disease amyloid β peptide. Biochimica et Biophysica Acta (BBA)-., Biomembranes, (2007), 1768, p1976-1990.
-
X. Wang, J. Cai, J. Zhang, C. Wang, A. Yu, Y. Chen, Z. Zuo, Acute trimethyltin exposure induces oxidative stress response and neuronal apoptosis in Sebastiscus marmoratus., Aquat. Toxicol, (2008), 90, p58-64.
[https://doi.org/10.1016/j.aquatox.2008.07.017]

- Ko. Wc, S.R. Lee, Effect of immature Citrus sunki peel extract on neuronal cell death., Hanguk Yakyong Changmul Hakhoe Chi, (2015), 23, p144-149.