Comparison of Anti-oxidant and Anti-inflammatory Activities of Methanolic Extracts Obtained from Different Parts of Cotoneaster wilsonii Nakai
This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The Ministry of Environment, Korea recognizes Cotoneaster wilsonii Nakai as a second-class endangered plant. It is a native species that grows in Ulleung-do, Korea. To our knowledge, the bioactivity of this plant has not yet been reported. Therefore, in this study, we have reported the bioactivity of C. wilsonii Nakai.
The anti-oxidant activities of C. wilsonii methanolic extract were investigated in vitro. The anti-oxidant activity was evaluated using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical assay, and the total phenolic and flavonoid content were measured. The leaf methanolic extract had the highest DPPH radical scavenging activity (IC50; 15.74 ㎍/㎖), and it also had the highest total phenolic and flavonoid content (220.95 ㎎·GAE/g, and 36.46 ㎎·QE/g extract respectively). Through high performance liquid chromatography (HPLC) analysis, chlorogenic acid was found to be the predominant among all phenolic compounds, showing a concentration of 84.24 ㎎/g extract. More than 60% decrease in nitric oxide (NO) production was found in the leaf methanolic extract.
To the best of our knowldege, this is the first report of the bioactivities of C. wilsonii. The results demonstrate that the leaf methanolic extract of C. wilsonii shows potent anti-oxidant, and anti-inflammatory activities.
Keywords:
Cotoneaster wilsonii Nakai, Anti-oxidant, Anti-inflammatory, Caffeic Acid, Chlorogenic acid, Ferulic Acid, Gallic Acid, High Performance Liquid Chromatography, Syringic AcidINTRODUCTION
Recently, there has been a lot of attention for the studies of reactive oxygen species (ROS). ROS cause aging and disease by breaking the balance with the anti-oxidant defense system in the human body (Nordberg et al., 2001; Duan et al., 2015; Yi, 2017). Research on natural anti-oxidants is actively conducted as medicinal plants used for treatment and prevention to remove free radicals (Bauer, 2000). ROS can be inhibited by phenolic and flavonoid compounds. Anti-oxidants materials can be used to prevent adult diseases and contribute to the delay and prevention of aging.
Currently, synthetic anti-oxidants with good effects such as butylated hydroxyanisole (BHA) and butylated hydroxy toluene (BHT) have been developed and used. However, problems have been reported that they are carcinogenic, toxic, disrupting reserved nutrient consumption and cell metabolism and breathing. Therefore, it is necessary to develop a natural antioxidant in plant origin that has a high anti-oxidant effect, safe and economical (Omaye, 1997).
Researchers suspect that the seeds of Cotoneaster multiflorus floating in the sea or carried by birds or winds may have adapted to the unusual environmental conditions of Ulleung-do and have been differentiated into C. wilsonii Nakai. The height of C. wilsonii Nakai is 1 to 4 meters tall. The leaves are irregular shape, oval or obovate, with narrow ends. Flowers bloom from May to June and the color is white. The sepals are white, furry at the end, and two petals are longer than stamen and have a style. It bears a fruit in September. It is red and egg-shaped (Kim et al., 2002).
Currently, the study of the C. wilsonii Nakai has been conducted up to plant characteristics, genetic diversity conservational status (Kim et al., 2002), a paper comparing flavonoids of East Asian Cotoneaster and C. wilsonii Nakai leaves (Chang and Jeon, 2003). Germination and other characteristics of seed (Lim et al., 2013) and so on. No studies on bioactivity have been conducted.
The main aims of this study was to provide scientific basis for the possibility of development as a natural product material by evaluating the effects of anti-oxidant, anti-inflammatory activities.
MATERIALS AND METHODS
1. Preparation of C. wilsonii Nakai extracts.
Methanolic extraction was provided by Gangwon Nature Environment Research park (Hongcheon, Korea) and used for this experiment. Dried leaves, stems, and fruits were extracted at room temperature by three times with 100% methanol. And, the methanolic extract was filtered and then evaporated using a vacuum rotary evaporator (N-1200A, Eyela, Tokyo, Japan) under reduced pressure at 40℃. It was stored at -70℃ in deep freezer.
2. Chemicals and reagents.
1,1-diphenyl-2-picrylhydrazyl (DPPH), ascorbic acid, gallic acid, α-tocopherol, quercetin, trichloroacetic acid, aluminium chloride, 2,6-di-tert-butyl-4-methylphenol (BHT), 3-tert-butyl-4-hydroxyanisole (BHA) used Sigma-Aldrich (St. Louis, MO, USA) products to measure anti-oxidant activities, and all other reagents were purchased and used with premium prouducts from Deajung (Siheung, Korea) and Junsei (Tokyo, Japan). HPLC grade water and acetonitrile was purchased from Avantor (Delaware, PA, USA).
Macrophages (RAW264.7) used in the experiment was obtained from Korean Cell Line Bank (Seoul, Korea). Phosphate buffered saline (PBS), 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenyl-formazan (MTT), lipopolysaccharides (LPS) used Sigma-Aldrich (St. Louis, MO, USA) products. Dulbecco’s modified eagle’s medium (DMEM), fetal bovine serum (FBS) and penicillin were purchased from biowest (Maine-et-Loire, France).
3. Determination of anti-oxidant activity.
DPPH radical scavenging activity was evaluated using the modifying method of Blois (1958). Zero point one ㎖ of the different concentrations extracts added with the 0.1 ㎖ of 0.15 mM 1,1-Diphenyl-2-picrylhydrazyl (DPPH). And incubated for 30 min at room temperature in dark. This was detected at 517 ㎚ using a UV/VIS spectrophotometer (V530, Jasco Co., Tokyo, Japan). BHA, BHT, α-tocopherol and ascorbic acid were used as positive controls. The concentration of the samples at the time of 50% inhibition of DPPH was represented by IC50.
Reducing power of C. wilsonii Nakai was determined according to modifying the method by Oyaizu (1986). Zero point one ㎖ of the diluted extracts using distilled water was added with 0.1 ㎖ of 0.2 M sodium phosphate buffer (pH 6.6), 0.1 ㎖ of 1% potassium ferricyanide, 0.1 ㎖ of 10% trichloroacetic acid and 0.4 ㎖ of distilled water. Added 0.05 ㎖ ferric chloride and then directly measured it using a UV/VIS spectrophotometer (V530, Jasco Co., Tokyo, Japan) on 700 ㎚.
4. Determination of total phenolic and total flavonoid contents.
Total phenolic content of C. wilsonii Nakai was determined according to modifying the Folin-Ciocalteau method of by Singleton and Rossi (1965). Zero point one ㎖ of the extracts was added to 0.05 ㎖ of Folin–Ciocalteu reagent and incubated for 3 min at room temperature. Then added the 0.3 ㎖ of 20% sodium carbonate. And after 15 min, added the 1 ㎖ of distilled water. The mixture was detected at 725 ㎚ using a UV/VIS spectrophotometer (V530, Jasco Co., Tokyo, Japan). The phenolic content was calculated using the linear equation based on the gallic acid calibration curve. And the results were expressed as the gallic acid equivalence per gram of extract (㎎·GAE/g).
Total flavonoid content of C. wilsonii Nakai. was determined according to modifying the method of by Nieva Moreno et al. (2000). Zero point one ㎖ of the diluted extracts using 80% EtOH was added with 0.02 ㎖ of 10% aluminum nitrate, 0.02 ㎖ of 1 M potassium acetate and 0.86 ㎖ 80% EtOH. After 40 min at room temperature, the mixture was detected at 415 ㎚ using a UV/VIS spectrophotometer (V530, Jasco Co., Tokyo, Japan). The flavonoid content was calculated using the linear equation based on the quercetin calibration curve. And the results were expressed as the querectin equivalence per gram of extract (㎎·QE/g).
5. MTT assay.
Cytotoxicity of the C. wilsonii Nakai to RAW264.7 was determined according to modifying the MTT method of Mosmann (1983). RAW264.7 macrophage cells were seeded in 96-well culture plate at 1 × 104 cells/well and incubated for 24 hr. And then, all medium was replaced 0.1 ㎖ of different concentrations of the samples (0 - 200 ㎍/㎖) and incubated for 24 hr. And the changed 0.1 ㎖ of 500 ㎍/㎖ of MTT. After 4 hr, 0.1 ㎖ of dimethyl sulfoxide (DMSO) was treated. After the treatment, it was detected at 540 ㎚ using a ELISA (Model 680, Bio-Rad, Hercules, CA, USA) microplate absorbance reader.
6. Determination of NO production of RAW264.7 macrophages.
NO production of C. wilsonii Nakai was determined according to modifying the Griess reagent system of by Green and Kroemer (1982). RAW264.7 macrophage cells were seeded in 96-well culture plate at 1 × 105 cells/well and incubated for 24 hr. Medium was replaced different concentrations of the samples (0 - 200 ㎍/㎖). After 30 min of the incubation, 2 ㎍/㎖ of LPS was added and incubated for 24 hr. After the treatment, 0.05 ㎖ supernatant mixed with 0.05 ㎖ Griess reagent (A reagent; 1% sulfanilamide, B reagent; 0.1% n-(1-naphthyl)-ethylenediamine dihydrochloride melted in 5% phosphoric acid) was detected at 540 ㎚ using a ELISA (Model 680, Bio-Rad, Hercules, CA, USA) microplate absorbance reader.
7. Determination of phenolic derivatives content in C. wilsonii Nakai.
Six phenolic derivatives were analyzed simultaneously using HPLC. Six phenolic derivatives were gallic acid, chlorogenic acid, caffeic acid, syringic acid, coumaric acid and ferulic acid. The HPLC analysis was analyzed using e2695 system of the Waters (Milford, MA, USA). The detector was 2489 UV-Vis detector of the Waters (Milford, MA, USA). The analytical column was a CAPCELL PAK C18 (5 ㎛, 4.6 ㎜ × 250 ㎜) of the Shiseido (Tokyo, Japan). The mobile phase was a mixture of 0.01% phosphoric acid in water and acetonitrile. The flow rate of mobile phase was 1.0 ㎖/min. The wavelength of UV detector was set at 272 ㎚.
8. Statistical analysis method.
Each data is means ± standard derivation (SD) from triplicate samples of three independent experiments. Overall differences among the treatment groups were determined using One-way analysis of variance (ANOVA) by IBM SPSS Statistic 24 Software (Armonk, NY, USA). p < 0.05 is regarded as significance.
RESULTS
1. Anti-oxidant activity of C. wilsonii Nakai.
The results of the DPPH radical scavenging activity, the IC50 values of the stem methanolic extract was 22.00 ± 0.14 ㎍/㎖, the leaf methanolic extract was 15.74 ± 0.16 ㎍/㎖ and the fruit methanolic extract was 80.58 ± 0.84 ㎍/㎖ (Table 1). The leaf methanolic extract showed the highest anti-oxidant effect and showed higher activity than BHT (147.56 ㎍/㎖) used as an antioxidant and showed activity similar to α-tocopherol (12.33 ㎍/㎖). The availability of C. wilsonii Nakai extract as antioxidant was confirmed.
The different part extracts of C. wilsonii Nakai were treated with 100, 250, 500, and 1,000 ㎍/㎖. Respectively, the reducing power was increased as the concentration increased. The leaf methanolic extract and stem methanolic extract showed the high reducing power effect. Effect was found similar to the ascorbic acid used as positive control when treated at 1,000 ㎍/㎖ concentrations (Fig. 1).
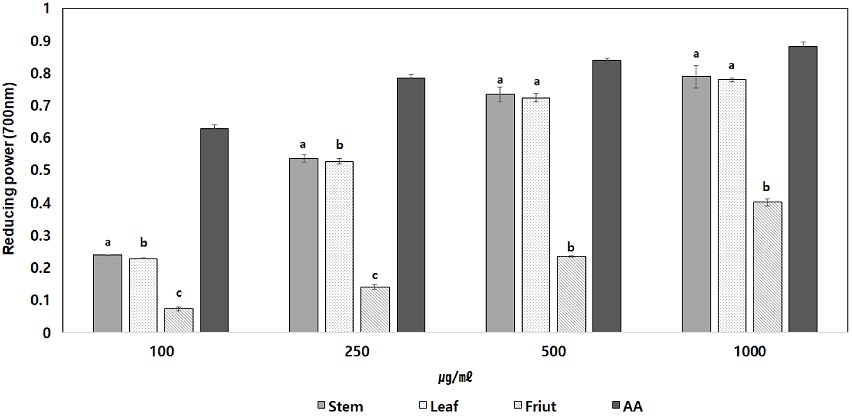
Reducing power of extractions from C. wilsonii Nakai.AA; Ascorbic acid. Each Data is means ± standard deviation of three replicate tests. The all data were statistically analyzed used by One-way analysis of variance (ANOVA). And differences were assessed that significant at 5% level of probability (p < 0.05).
This supports the correlation between anti-oxidant activity and reducing power reported in many studies, as well as results such as Kim et al. (2013) similar to DPPH inhibition assay.
2. Total phenolic and total flavonoid content of C. wilsonii Nakai.
The total phenolic content of extracts from different parts in C. wilsonii Nakai was high in order of leaf methanolic extract (220.95 ± 0.50 ㎎·GAE/g) > stem methanolic extract (214.65 ± 2.43㎎·GAE/g) > fruit methanolic extract (60.71 ± 2.03 ㎎·GAE/g). And the total flavonoid content was also the highest in leaf methanolic extract with 36.46 ± 1.89 ㎎·QE/g (Table 2). This supports the results that total phenolic and total flavonoid content was involved in anti-oxidant activity reported by Rice-Evans et al. (1996) and Zou et al. (2012), along with DPPH inhibition assay results, and indicates the high antioxidant effects of the C. wilsonii Nakai extract.
3. Effect of C. wilsonii Nakai on NO production in LPS-stimulated RAW264.7 macrophages.
An experiment was conducted using the macrophage RAW264.7 to determine the anti-inflammatory activity of the C. wilsonii Nakai extract. For the determination of cytotoxicity, various concentrations of extracts (10, 50, 100, 200 ㎍/㎖) were treated. Stem methanolic extract showed a survival rate of less than 20% at 50 ㎍/㎖, while leaf methanolic extract produced a survival rate of 67% at 200 ㎍/㎖, and fruit methanolic extract produced a survival rate of 43% (Fig. 2).
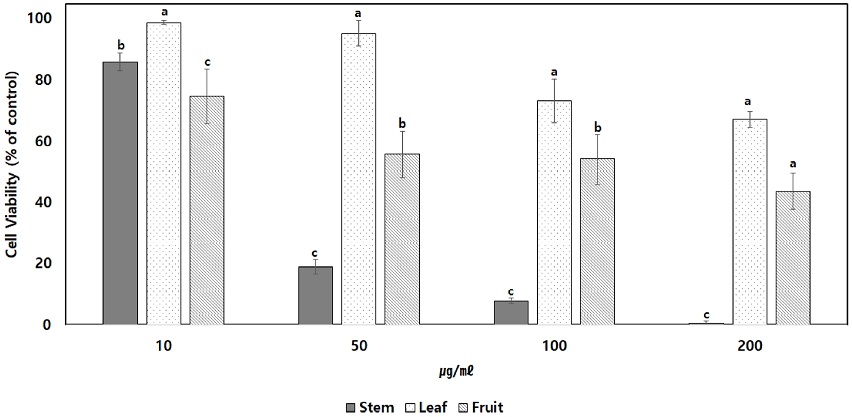
Inhibitory effect of extract from C. wilsonii Nakai on cytotoxicity of RAW264.7 cell. Each Data is means ± standard deviation of three replicate tests. The all data were statistically analyzed used by One-way analysis of variance (ANOVA). And differences were assessed that significant at 5% level of probability (p < 0.05).
RAW264.7 cells were treated with extracts at various concentrations (10, 50, 100, 200 ㎍/㎖) and NO production was measured by stimulating LPS, an inflammatory substance. As a result, NO production was reduced in proportion to the concentration in leaf methanolic extract (Fig. 3). Stem methanolic extract was unable to confirm its effect due to cytotoxicity. RAW264.7 effectively inhibited NO production within cells, confirming that the C. wilsonii Nakai leaf methanolic extract is available as a natural substance to anti-inflammatory.
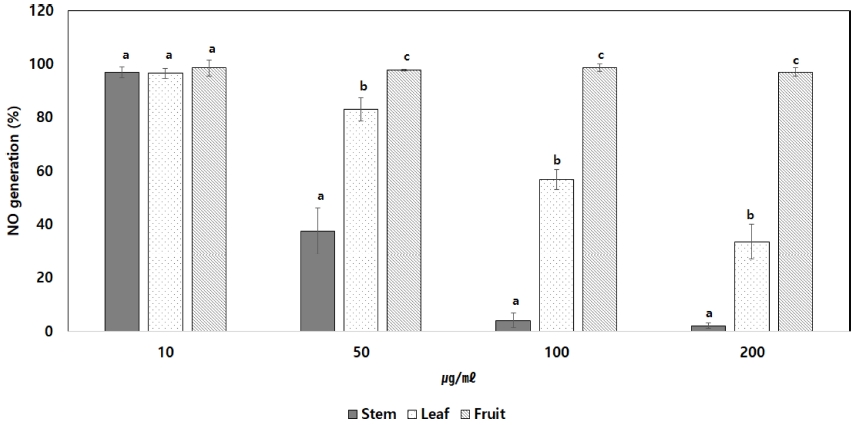
The effect of C. wilsonii Nakai on LPS-induced NO production in RAW264.7 cell. Each Data is means ± standard deviation of three replicate tests. The all data were statistically analyzed used by One-way analysis of variance (ANOVA). And differences were assessed that significant at 5% level of probability (p < 0.05).
4. Phenolic derivatives contents of the C. wilsonii Nakai using HPLC.
It was estimated that the anti-oxidant and anti-inflammatory effect’s substance in C. wilsonii Nakai extract was phenolic derivatives. Therefore, phenolic derivatives were analyzed using HPLC. In Fig. 4, six phenolic derivatives and extracts by different parts of the C. wilsonii Nakai were shown the chromatogram by HPLC. Five phenolic derivatives were detected in leaf methanolic extract, three phenolic derivatives detected in stem methanolic extract and two phenolic derivatives detected in fruit methanolic extract.
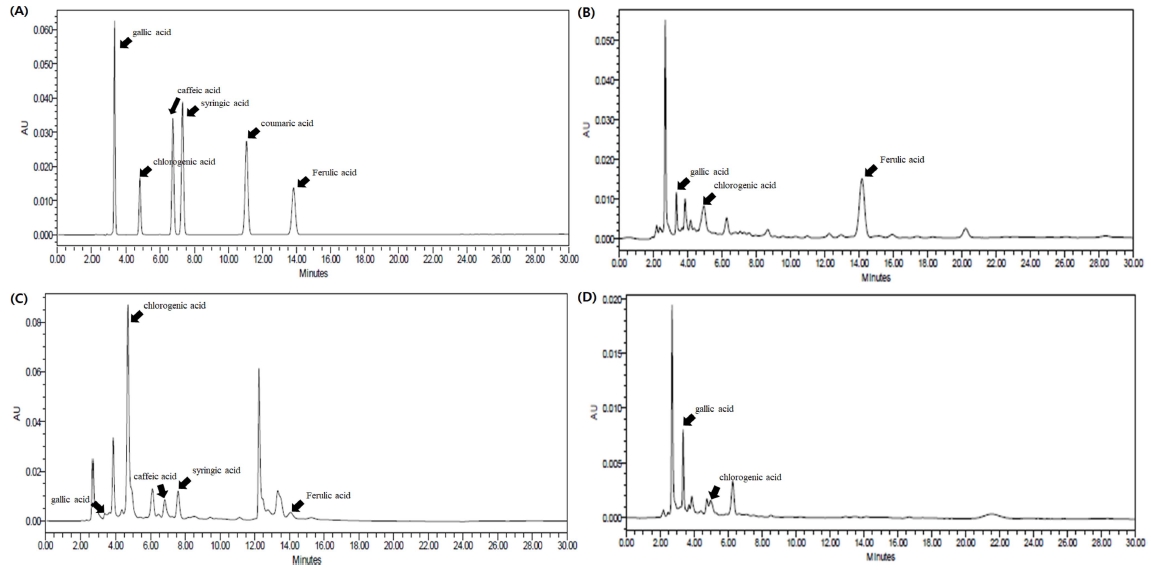
HPLC chromatograms of standard compounds and methanolic extracts from C. wilsonii Nakai. (A); standard compounds, (B); stem methanolic extract, (C); leaf methanolic extract, (D); fruit methanolic extract.
The calibration curve and limit of detection (LOD), limit of quantitation (LOQ) for each standard for quantifying the content of detected compounds are summarized in Table 3. The calibration curve and correlation coefficient (R2) were calculated using four concentrations (10, 25, 50 and 100 ㎍/㎖). LOD, LOQ was obtained by signal-to-noise (S/N). LOD was calculated as the S/N value of 3 : 1. And. LOQ was calculated as the S/N value of 10 : 1. The quantification results are shown in Table 4. This similar to the previous experiment of total phenolic content, the most contents in leaf methanolic extract was detected. Similar to the total phenolic content experiment that previously tested, the largest contents was detected in leaf methanolic extract, and in order to high chlorogenic acid (84.24 ± 0.64 ㎎/g·Ext.) > syringic acid (1.99 ± 0.01 ㎎/g Ext.) > ferulic acid (1.71 ± 0.04 ㎎/g·Ext.) > caffeic acid (1.67 ± 1.44㎎/g·Ext.) > gallic acid (1.19 ± 0.02㎎/g·Ext.).
This results supports that the total phenolic content is involved in anti-oxidant activity reported by Rice-Evans et al. (1996) and is involved in anti-inflammatory by Elizabeth et al. (2005).
DISCUSSION
DPPH radical inhibition assay, total phenolic content and total flavonoid content, reducing power assay, HPLC qualitative and quantitative analysis were conducted to review the availability of natural antioxidants through anti-oxidant activity effect about C. wilsonii Nakai. According to the results, high anti-oxidant activity was found in leaf methanolic extract.
The IC50 value of 15.74 ± 0.16 ㎍/㎖ in DPPH radical inhibition assay showed higher activity than the BHT used as an anti-oxidant and similar activity to α-tocopherol. Total phenolic and flavonoid content were 220.95 ± 0.50 ㎎·GAE/g, 36.46 ± 1.89 ㎎·QE/g, HPLC analysis showed that chlorogenic acid was the highest with 84.24 ± 0.64 ㎎/g·Ext. The antioxidant activity in C. wilsonii Nakai has not been reported yet, and the anti-oxidant effect is excellent.
When compared with the C. multilorus fruit which is believed to have been transferred and separated into C. wilsonii Nakai on Ulleung-do, It is reported that DPPH radical inhibition assay IC50 could not be found in the ethanol-water (7 : 3, v/v) extract of C. multilorus fruit in a scientific paper (Liu et al., 2018). Thus, the extracts of the C. wilsonii Nakai fruit studied in this paper showed a higher anti-oxidant effect. And C. integererimus (Uysal et al., 2016), which a plant of the same genus Cotoneaster, has been reported that the phenolic content was 115.15 ㎎·GAE/g and flavonoid content was 16.29 ㎎/g in twing methanolic extract. Therefore, it was confirmed that the phenol content (214.65 ㎎·GAE/g) is higher in the stem methanolic extract of the C. wilsonii Nakai.
Inflammation is the cause of chronic diseases such as obesity, cardiovascular disease, metabolic syndrome and dementia (Chung et al., 2009). Measurement of NO generation, an inflammatory substance, showed that the amount of NO produced in RAW264.7 cells was dependently reduced on concentration compared to negative control in leaf methanolic extract. In particular, NO production was reduced by 67% at 200 ㎍/㎖.
This result is attributed that chlorogenic acid is high in the leaf methanolic extract (84.24 ± 0.64 ㎎/g·Ext.). This supports the results that chlorogenic acid is involved in anti-inflammatory by Hwang et al. (2014). For stem methanolic extract, RAW264.7 cells were found to be toxic (Fig. 2) and fruit methanolic extract were found to be ineffective in the NO generation (Fig. 3).
This is consistent with the results that inflammatory reactions are effectively inhibited if oxidative stress is blocked by an anti-oxidant reported by several researchers (Kim et al., 2011; Kim et al., 2015). Therefore, the anti-inflammatory effect of the C. wilsonii Nakai is thought to be due to the strong antioxidant power of the C. wilsonii Nakai as aforementioned.
The results of this study are the first report of anti-oxidant effect in the C. wilsonii Nakai. Comparing with the same Cotoneaster plants, it has been found that the high anti-oxidant effect. The leaf extract of the C. wilsonii Nakai is thought to be available as a natural antioxidant. Further research on bioactivities is required for the possibility of development as a natural material of the C. wilsonii Nakai.
References
- Bauer, G., (2000), Reactive oxygen and nitrogen species: Efficient, selective, and interactive signals during intercellular induction of apoptosis, Anticancer Research, 20, p4115-4139.
-
Blois, MS., (1958), Antioxidant determinations by the use of a stable free radical, Nature, 181, p1199-1200.
[https://doi.org/10.1038/1811199a0]

-
Chang, CS, and Jeon, JI., (2003), Leaf flavonoids in Cotoneaster wilsonii(Rosaceae) from the island Ulleung-do, Korea, Biochemical Systematics and Ecology, 31, p171-179.
[https://doi.org/10.1016/s0305-1978(02)00064-9]

-
Chung, HY, Cesari, M, Anton, S, Marzetti, E, Giovannini, S, Seo, AY, Christy, C, Carter, C, Yu, BP, and Leeuwenburgh, C., (2009), Molecular inflammation: Underpinnings of aging and age-related diseases, Ageing Research Reviews, 8, p18-30.
[https://doi.org/10.1016/j.arr.2008.07.002]

-
Duan, Y, Kim, GH, Seong, JH, Chung, HS, and Kim, HS., (2015), Antioxidant activities of n-butanol and ethyl acetate extracts from Yam(Dioscorea batatas Decne), Journal of the Korean Oil Chemists’ Society, 32, p599-606.
[https://doi.org/10.12925/jkocs.2015.32.4.599]

-
Elizabeth A. Miles, Zoubouli, P, Calder, P, and Phil, D., (2005), Differential anti-inflammatory effects of phenolic compounds from extra virgin olive oil identified in human whole blood cultures, Nutrition, 21, p389-394.
[https://doi.org/10.1016/j.nut.2004.06.031]

-
Green, DR, and Kroemer, G., (2005), Pharmacological manipulation of cell death: Clinical applications in sight?, Journal of clinical investigation, 115, p2610-2617, (cited by 2019 April 8).
[https://doi.org/10.1172/JCI26321]

-
Hwang, SJ, Kim, YW, Park, YH, Lee, HJ, and Kim, KW., (2014), Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW264.7 cells, Inflammation Research, 63, p81-90.
[https://doi.org/10.1007/s00011-013-0674-4]

-
Kim, CS, Kim, J, Lee, YM, Sohn, E, Jo, K, and Kim, JS., (2011), Inhibitory effects of chlorogenic acid on aldose reductase activity in vitro and cataractogenesis in galactose-fed rats, Archives of Pharmacal Research, 34, p847-852.
[https://doi.org/10.1007/s12272-011-0519-z]

-
Kim, HS, Kim, MS, Kim, SH, Yun, KW, and Song, JH., (2013), Analysis of total phenolic content and antioxidant activity from fruits of Vaccinium oldhamii Miq, Journal of Korean Society of Forest Science, 102, p566-570.
[https://doi.org/10.14578/jkfs.2013.102.4.566]

-
Kim, YH, Cho, ML, KIm, D, Shin, GH, Lee, JH, Lee, JS, Park, SO, Lee, SJ, Shin, HM, and Lee, OH., (2015), The antioxidant activity and their major antioxidant compounds from Acanthopanax senticosus and A. koreanum, Molecules, 20, p13281-13295.
[https://doi.org/10.3390/molecules200713281]

-
Kim, YS, Chang, CS, Shin, HT, Kim, H, and Choi, DY., (2002), Conservational status of Cotoneaster wilsonii on island Ulleung-do, Korean Journal of Plant Taxonomy, 32, p159-175.
[https://doi.org/10.11110/kjpt.2002.32.2.159]

-
Lim, JS, Park, SY, Lim, JO, and Lee, BW., (2013), A faunastic study of insects from is. Ulleungdo and its nearby islands in South Korea, Journal of Asia-Pacific Biodiversity, 6, p93-121.
[https://doi.org/10.7229/jkn.2013.6.1.093]

-
Liu, XP, Jia, J, Jing, XM, and Li, GI., (2018), Antioxidant activities of extracts from sarcocarp of Cotoneaster multiflorus, Journal of Chemistry, 2018, 4619768 (cited by 2019 May 20).
[https://doi.org/10.1155/2018/4619768]

-
Mosmann, T., (1983), Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays, Journal of Immunological Methods, 65, p55-63.
[https://doi.org/10.1016/0022-1759(83)90303-4]

- Nieva Moreno, MI, Isla, MI, Sampietro, AR, and Vattuone, MA., (2000), Comparison of the free radical-scavenging activity of propolis from several regions of Argentine, Journal of Ethnopharmacology, 71, p109-114.
- Nordberg, J, and Arnér, ESJ., (2001), Reactive oxygen species, antioxidants, and the mammallan thioredoxin system, Free Radical Biology and Medicine, 31, p1287-1312.
- Omaye, ST, Reddy, KA, and Cross, CE., (1997), Effect of butylated hydroxytoluene and other antioxidants on mouse lung metabolism, Journal of Toxicology and Environmental Health, 3, p829-836.
-
Oyaizu, M., (1986), Studies on products of browning reactions: Antioxidative activities of products of browning reaction prepared from glucosamine, Japanese Journal of Nutrition, 44, p307-315.
[https://doi.org/10.5264/eiyogakuzashi.44.307]

-
Rice-Evans, CA, Miller, NJ, and Paganga, G., (1996), Structure-antioxidant activity relationships of flavonoids and phenolic acids, Free Radical Biology and Medicine, 20, p933-956.
[https://doi.org/10.1016/0891-5849(95)02227-9]

- Singleton, VL, and Rossi, JA., (1965), Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents, American Journal of Enology and Viticulture, 16, p144-158.
-
Uysal, A, Zengin, G, Mollica, A, Gunes, E, Locatelli, M, Yilmaz, T, and Aktumsek, A., (2016), Chemical and biological insights on Cotoneaster integerrimus: A new(-)-epicatechin source for food and medicinal applications, Phytomedicine, 23, p979-988.
[https://doi.org/10.1016/j.phymed.2016.06.011]

- Yi, MR, Kang, CH, and Bu, HJ., (2017), Antioxidant and anti-inflammatory activity of extracts from kohlrabi(Brassica Oleracea var. Gonglodes), Journal of the Korean Oil Chemists’ Society, 34, p189-202.
-
Zou, Y, Liao, S, Shen, W, Liu, F, Tang, C, Chen, CYO, and Sun, Y., (2012), Phenolics and antioxidant activity of mulberry leaves depend on cultivar and harvest month in southern China, International Journal of Molecular Sciences, 13, p16544-16553, (cited by 2019 May 15).
[https://doi.org/10.3390/ijms131216544]