Purification and Identification of Antioxidant Compounds from Dolichos lablab L. Seeds
 ; Jae Yeon Kim2
; Jae Yeon Kim2 ; Yong Beom Cho3
; Yong Beom Cho3 ; Bang Yeon Hwang4
; Bang Yeon Hwang4 ; Jun Gu Kim5
; Jun Gu Kim5 ; Sun Hee Woo6
; Sun Hee Woo6 ; Moon Soon Lee7, †
; Moon Soon Lee7, †
This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
This study aimed to identify antioxidant compounds from the seeds of Dolichos lablab L. by bioassay-guided isolation and recrystallization.
The water layer of D. lablab L. seed extract inhibits intracellular reactive oxygen species (ROS) expressing the 2’,7’-dichlorofluorescein diacetate (DCF-DA), Cu/Zn superoxide dismutase (SOD) and catalase genes, as determined by quantitative real-time PCR (qRT-PCR). Two compounds were purified from the water layer of the seeds of D. lablab L. using column chromatography and prep-high performance liquid chromatography (HPLC). Using nuclear magnetic resonance (NMR) and electrospray Ionization mass spectrometry (ESI-MS), their chemical structures were identified as 5-[(2-acetyl-2,3-dihydro-1H-indazol-1-yl)carbonyl]-4,5-dihydro-3H–furan-2-one (C14H14N2O4) and stachyose.
Two active antioxidant compounds were purified from the seed extract of D. lablab L. seed extract and the structures of these compounds were identified as C14H14O4N2 and stachyose.
Keywords:
Dolichos lablab L., Chemical Structures, Reactive Oxygen Species Inhibitory Effect, Seed Extract, C14H14O4N2, StachyoseINTRODUCTION
Dolichos lablab L. is a leguminous species widespread throughout the tropics. Legumes are important sources of proteins, dietary fiber, carbohydrates, and minerals consumed worldwide. These legumes might be of importance in many zones of developing countries where there is a pressing need for food sources of good protein quality and high energy (Osman, 2007).
The D. lablab L. seeds classified by the National Academy of Science, USA as potential source of the protein that have not been explored yet. D. lablab is used when there is coldness like a cooling disease, diarrhea and abdominal pain etc. when it is hot weather in summer and plenty of rainy season in oriental medicine.
Phytochemical analysis of D. lablab was showed that it contained sugar, phenols, alkaloids, tannins, saponins, terpenoids, steroids, flavonoids, and many other metabolites (Al-Snafi, 2017). D. lablab has been studies by some researcher, such as hypolipidemic and hepatoprotective effect from water extract (Ramakrishna et al., 2007; Im et al., 2016), anti-diabetic activity from methanol (MeOH) extract (Kante and Reddy, 2013), and anti-microbial activity from n-hexane extract of leaf and flower (Priya and Jenifer, 2014). And dolichin purified from D. lablab inhibited Human Immunodeficiency Virus (HIV) reverse transcriptase and α- and β-glucosidase which are glycohydrolase implicated in HIV infection (Ye et al., 2000).
Major intracellular reactive oxygen species (ROS) produced are hydroxyl radical, superoxide anion, and hydrogen peroxide (Salvemini et al., 2003). Low intracellular ROS is an important mechanism of pathogen killing and also leads to endothelial damage resulting in an increased vascular permeability and cell death (Tiidus, 1998). However, intracellular ROS production plays important role in modulation of release of other inflammatory mediators. This is related mainly to the constitutive expression of NAD(P)H oxidases in various tissues (Bedard and Krause, 2007). ROS produced by this family of enzymes can regulate adhesion molecule expression on endothelium and inflammatory cells, so affecting cell recruitment to the sites of inflammation (Niu et al., 1994). They also increase cytokine expression and chemokine (Brzozowski et al., 2003). At least part of these effects results from the ability of ROS to stimulate MAPK and NF-kB activity which leads to activation of several transcription factors. It is possible that intracellular ROS may act as second messengers in inflammatory signal transduction (Guzik et al., 2003).
In this study, we analyzed antioxidant activity by lipopolysaccharide (LPS)-stimulated RAW264.7 cells in D. lablab extract and segregated substances that exhibit antioxidant activity via open column, high-performance liquid chromatography (HPLC). And the structure of substances was identified by electrospray ionization-mass spectrometry (ESI-MS) and nuclear magnetic resonance (NMR). Therefore, we intend to have value as a basic research to discover pharmacology resources with excellent antioxidant effect.
MATERIALS AND METHODS
1. Plant material
The seed of Dolichos lablab L. was purchased from commercial, and used for experiment after being dried in the shade.
2. Extraction and fractionation
The dried seed of D. lablab (1 ㎏) were ground, and extracted with 10 ℓ water three times at 65℃ (each time for 6 hours). The combined water extract was concentrated in vacuo at 40℃ to yield 250 g of residue. The water extract was kept refrigerated at 4℃. And the concentrated extract was resuspended in water and then partitioned successively with diethylether (Et2O), ethyl acetate (EtOAc), and n-butanol (n-BuOH).
The partitioned water (H2O) layer was concentrated in vacuo at 40℃. The H2O layer afforded precipitate on standing at a cold chamber. A portion of the extract was subjected to column chromatography [glass column (3 ㎝ × 35 ㎝)] packed with Diaion HP-20 (particle size 250 ㎜, Supelco, Bellefonte, PA, USA) using MeOH gradient (0% to 100%) and water finally as eluent. The 21 fractions were obtained from this initial column chromatographic separation, and then combined to two fractions (A and B) by using thin layer chromatography (TLC) [chloroform : MeOH : water = 60 : 30 : 6 (v/v)]. The active fraction A was rechromatographed on silica gel column (3 ㎝ × 35 ㎝) using the same mixture as eluent to give 16 fractions, and then combined to three factions by using TLC.
3. Purification and identification
Compound I and II from the fraction were purified using an HPLC system, which is consisting of C18 column (4.6 ㎜ × 250 ㎜ I.D, particle size 5 ㎜, SHISEIDO Inc., Osaka, Japan), UV detector 254 ㎚, flow rate 1 ㎖/min, and 1% MeOH as eluent, and the recrystallized with MeOH. Identification of compound I and II were assessed by NMR and ESI-MS.
1H- and 13C-NMR spectra were recorded in deuterium oxide (D2O) at 500 ㎒ and 100 ㎒, respectively. To confirm the molecular weight of two compounds, we measured the ESI-MS under the same conditions as the NMR. The two compounds confirmed the molecule by comparing the NMR data and the ESI-MS data.
4. Experimental cells
The RAW264.7 cells were purchased from Korea Cell Line Bank (KCLB, Seoul, Korea). They were cultured in Dulbecco’s modified eagle’s medium (DMEM, GibcoTM, Thermo Fisher Scientific, Waltham, MA, USA) and supplemented with 10% fetal bovine serum (FBS, Seradigm, Radnor, PA, USA), 1% penicillin-streptomycin (Sigma-Aldrich Co., St. Louis, MN, USA) in humidified 5% CO2 incubator at 37℃. The use of cells was carried out in accordance with the guide for the care and use of cells, Chungbuk National University, Korea.
5. Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from RAW264.7 cells treated with extracts in the presence or absence of LPS (1 ㎎/㎖) using TRI reagent (Molecular Research Center Inc., Cincinnati, OH, USA). Complementary DNA (cDNA) was synthesized using the ReverTra Ace® qPCR RT Master Mix with qDNA Remover (TOYOBO Co., Ltd., Osaka, Japan) according to the manufacturer’s instructions. qRT-PCR was performed using the SYBR® Green Real-time PCR Master Mix (TOYOBO Co., Ltd., Osaka, Japan) in the CFX96TM Real-time system (Bio-Rad Inc., Herules, CA, USA) with default parameters. Information of primer pairs in qRT-PCR are listed in Table 1.
6. Intracellular ROS measurement
Intracellular oxidative stress was detected using 2’,7’-dichlorofluorescein diacetate (DCF-DA) by Warleta et al. (2011). RAW264.7 cells treated with or without LPS in the presence of compounds for 24 h were washed twice with Hank’s buffered salt solution (HBSS) and incubated with 20 mM of DCF-DA in HBSS for 30 min at 37℃ in a CO2 incubator. Following wash with PBS, DCF fluorescence was measured at an excitation wave length of 485 ㎚ and emission at 525 ㎚. Since then the reaction was continued for an additional 30 min, and another measurement was performed. The intracellular ROS level was calculated as follows:
F = [(A30−A0)/A0]] × 100
Where A0 is the fluorescence before incubation and A30 is the fluorescence after incubation for 30 min.
7. Statistical analysis
All the experiments were reiterated at three independent replicates, and the results were presented as means ± standard deviation. Statistical significance was determined by Duncan’s Multiple Range Tests (DMRT) using SPSS 12.0 windows version (SPSS Inc., Chicago, IL, USA). Differences at p < 0.05 were considered significant.
RESULTS AND DISCUSSION
1. Identification of compound I and II
Identification of compound I and II were assessed by NMR and ESI-MS. 1H- and 13C-NMR spectra were recorded in D2O at 500 ㎒ and 100 ㎒, respectively. To confirm the molecular weight of two compounds, we measured the ESI-MS under the same conditions as the NMR. The two compounds confirmed the molecule by comparing the NMR data and the ESI-MS data.
According to the proton NMR result, the peaks of 8 ppm - 9 ppm on proton NMR data were indicated proton of aromatic ring and the area of proton NMR peak is equal to the number of protons. Therefore, the peaks of 8.73, 8.74, 8.75 and 9.03 ppm on proton NMR represent four protons in the aromatic of compound I. Based on the fact that the chemical shift was present and the area value of one unbroken peak displayed in peak of 2.04 ppm are three, it is considered that a methyl substituent is present.
And it was confirmed that it is a methyl substituent attached to acetyl group based on 13C-NMR and 2D-NMR. The following is the carbon NMR result. Based on the number of carbon NMR peaks, we confirmed that a total of 14 carbons are present. The three peaks of 173 ppm - 177 ppm were indicated three carboxyl group. Considering the chemical shift, we confirmed that the six peaks of 127 ppm - 147 ppm was the six carbons of benzene ring. And as a result of further performing 2D-NMR, the 14.51 peak was a methyl substituent attached to acetyl group. The carbon peaks of 26 ppm - 54 ppm were confirmed methylene and methine group.
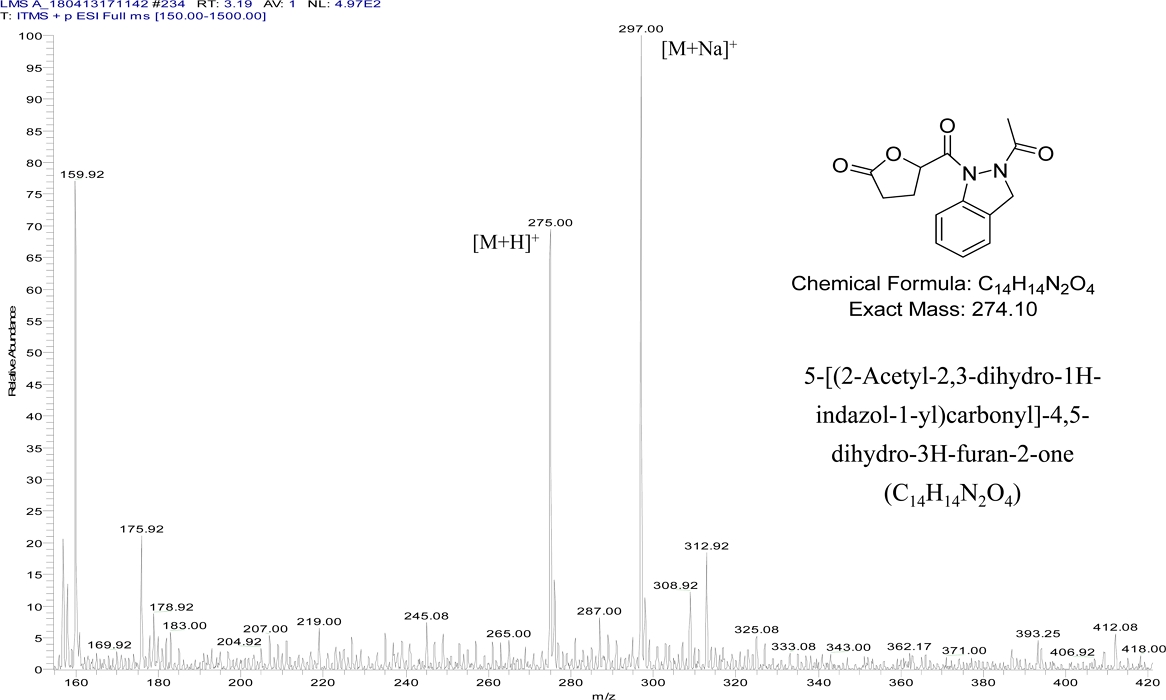
And as result MS spectrum, the molecular weight of compound I was 274 (Fig. 1). Based on determined 274 molecular weights by MS spectrum and predicted result by NMR, we confirmed that the molecular formula of compound I was identified to 5-[(2-acetyl-2,3-dihydro-1H-indazol-1-yl)carbonyl]-4,5-dihydro-3H–furan-2-one (C14H14N2O4) (Fig. 1 and Table 2).
According to the proton NMR result of the compound II, we confirmed anomeric peaks which are specific peaks in the sugar. The proton number 1 of sugar had a characteristic that when it is a cyclic structure, it comes out at ppm higher than other peaks due to the oxygen located on both sides. Therefore, based on the peaks of 5.35 ppm and 4.91 ppm, the structure of compound II was expected to be a structure with two or more sugar chains. In addition, the area of each peak was 1 : 2, and it was expected that two types of sugar would be connected at a ratio of 1 to 2. Peaks at 3.4 ppm - 4.1 ppm were identified as oxygenated methylene and methine proton, indicating that the peaks are divergent due to complex interactions within the cyclic structure of the sugar. The following is the carbon NMR result. In carbon NMR, anomeric carbons of the sugar were identified. Unlike general methylene and methine, the carbon peaks of 91.98, 97.87, 98.23, and 103.68 at high ppm are thought to be oxygenated anomeric carbon on both sides. In proton NMR, there are three anomeric carbons, whereas there are four anomeric carbons in carbon NMR. Therefore, compound II was identified that fructose without anomeric carbon was presented.
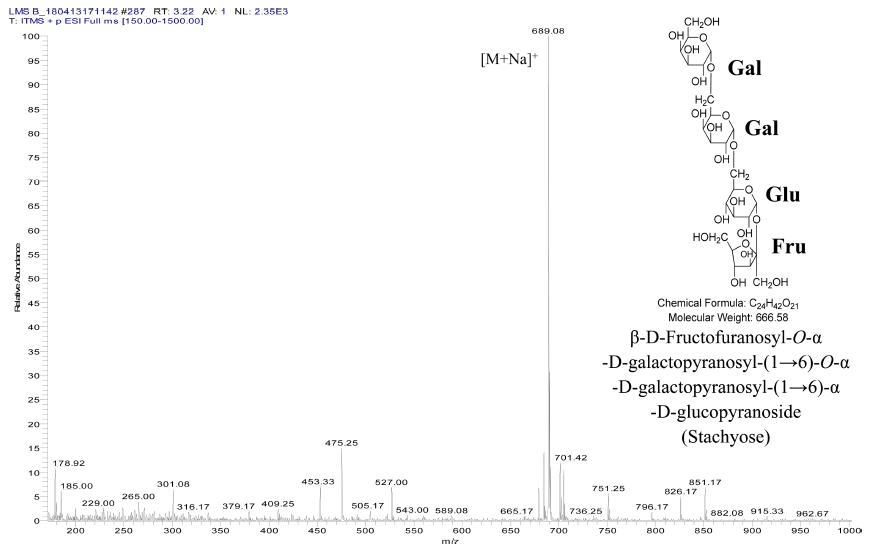
Based on determined 666 molecular weights by MS spectrum (Fig. 2) and predicted result by NMR, we confirmed that compound II was identified to β-D-fructofuranosyl-O-α-D-galactopyranosyl-(1→6)-O-α-D-galactopyranosyl-(1→6)-α-D-glucopyranoside (stachyose) (Fig. 2 and Table 3).
2. Antioxidant effect of compound I and II
To confirmed antioxidant activity of two compounds, we confirmed intracellular ROS inhibitory effect through DCF-DA fluorescence and copper/zinc superoxide dismutase (Cu/Zn SOD) and catalase genes expression through qRT-PCR. Cu/Zn SOD and catalase are enzymes that remove ROS in cells (Michiels et al., 1994).
We examined the gene expression levels of catalase and Cu/Zn SOD in LPS-treated RAW264.7 cells. The results were shown in Fig. 3. Although the two compounds were not significantly different towards the Cu/Zn SOD expression level, the expression level of catalase genes were indicated an increase rate of about 3.5 times in compound I and about 2.4 times in compound II at 50 ㎍/㎖ compared with LPS-not treated cells.
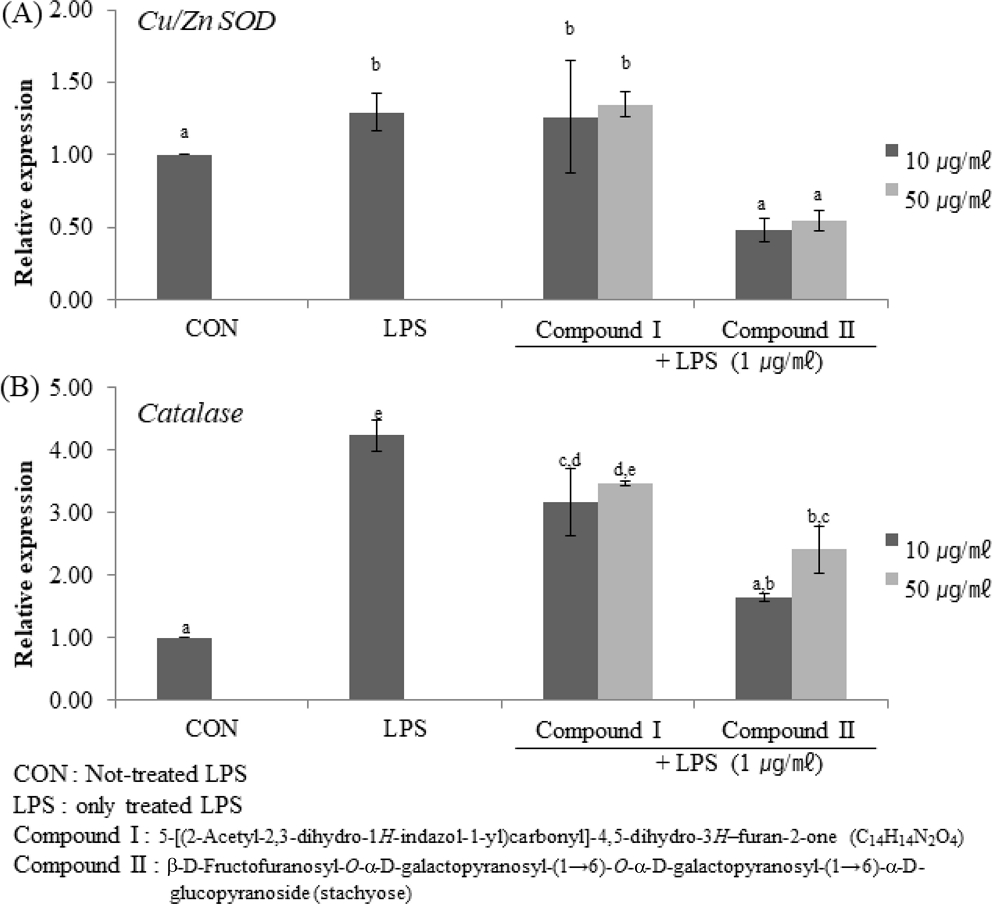
Effect of Compound I and II on the expression of LPS-induced SOD and catalase genes.(A) is the measured value of Cu/Zn SOD genes expression through qRT-PCR. (B) is the measured value of catalase genes expression through qRT-PCR. Mean values ± SE from triplicate separated experiments are shown. *Mean within a column followed by same letter are not significant based on the Duncan's Multiple Range Test (DMRT) (p < 0.05).
However, the enzyme genes levels of LPS-only treated cells were also increased. It was signified that the stress-affected cells are higher levels of enzyme genes expression than it is not. So, we determined intercellular ROS via DCF-DA fluorescence. The result was shown in Fig. 4. It was shown that two compounds inhibited intercellular ROS levels to dose-dependent in LPS-treated RAW264.7 cells.
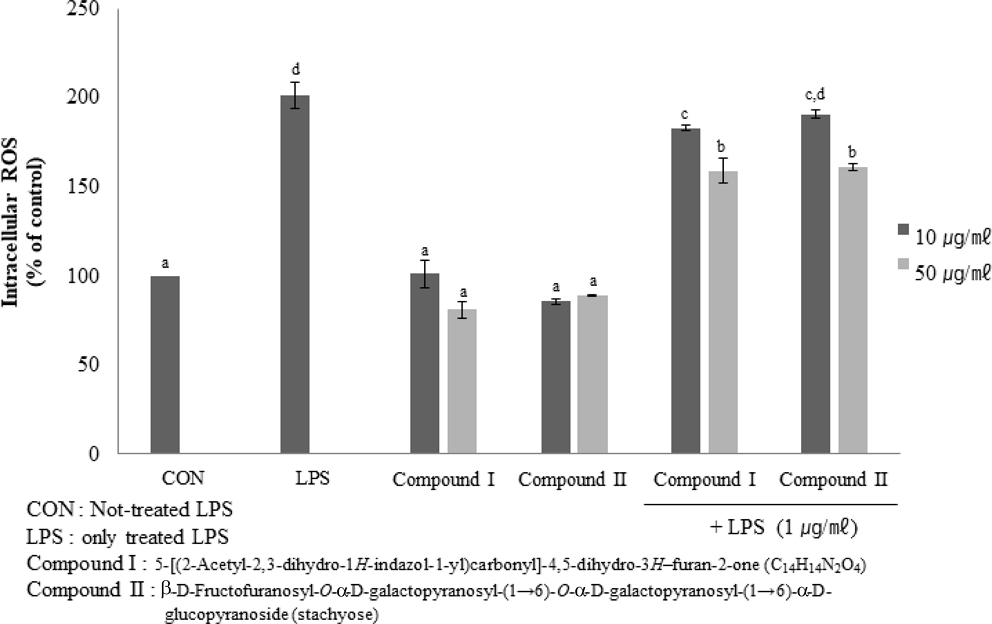
Effect of Compound I and II on antioxidant activity on LPS-induced RAW264.7 cells.Mean values ± SE from triplicate separated experiments are shown. *Mean within a column followed by same letter are not significant based on the Duncan's Multiple Range Test (DMRT) (p < 0.05).
And through two compounds inhibited intracellular ROS in LPS-not treated RAW264.7 cells, it was confirmed that two compounds did not affect as stress in cells.
Stachyose, β-D-fructofuranosyl-O-α-D-galactopyranosyl-(1→6)-O-α-D-galactopyranosyl-(1→6)-α-D-glucopyranoside, belong to the raffinose family oligosaccharides (RFOs). Physiologically, RFOs in seed may be regarded as storage carbohydrates. They are broken down rapidly during the early stages of germination and may thus provide readily available energy and substrates to support growth (Peterbauer and Richter, 2001). Non-enzymatic antioxidant mechanisms in plant include polyphenols, glutathione, ascorbate, flavonoids, carotenoids, α-tocopherol and sugar-phenols and soluble carbohydrates, such as fructans and RFOs (Stoyanova et al., 2011). Also, stachyose indicated to act as an antioxidant in Arabidopsis seed (Nisizawa-Yokoi et al., 2008). and inhibited the human epithelial cell line Caco-2 proliferation and induced apoptosis (Huang et al., 2015). Therefore, we presumed that extracted starchyose in seed of D. lablab was indicated antioxidant activity.
Compound I, 5-[(2-acetyl-2,3-dihydro-1H-indazol-1-yl) carbonyl]-4,5-dihydro-3H–furan-2-one, form the basic structure by indazole. Indazole was defined by scientist Emil Fisher as a “pyrazole ring fused with the benzene ring”. Indazole nucleus is present in naturally occurring alkaloids and biologically active molecules. In recent years, some of the indazole ring systems are being evaluated as potential drugs for variety of physiological activities with at least few compounds approved for clinical use (Gaikwad et al., 2015). A large number of synthetically prepared indazole derivatives have displayed biological and pharmacological properties (Rahman et al., 1995). Such as Bendazac is a non-steroidal anti-inflammatory agent, used as an anti-cataract drug (Cerecetto et al., 2005). New YC-1 indazole derivative were synthesized and evaluated with hypoxia inducible factor (HIF)-1 transcriptional activity, in vivo (Takeuchi et al., 2012). Thus, we are regarded that extracted compound I in D. lablab also shows prominent activity against several diseases.
In conclusion, two antioxidant active compounds were purified from D. lablab L. seed extract and the structure was identified as 5-[(2-acetyl-2,3-dihydro-1H-indazol-1-yl)carbonyl]-4,5-dihydro-3H–furan-2-one and stachyose, respectively.
Acknowledgments
This work was carried out with financially supported by the Research Year of Chungbuk National University, Republic of Korea in 2019.
References
-
Al-Snafi AE. (2017). The pharmacology and medical importance of Dolichos lablab(Lablab purpureus): A review. IOSR Journal of Pharmacy. 7:22-30.
[https://doi.org/10.9790/3013-0702012230]

-
Bedard K and Krause KH. (2007). The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiological Reviews. 87:245-313.
[https://doi.org/10.1152/physrev.00044.2005]

-
Brzozowski T, Konturek PC, Konturek SJ, Kwiecień S, Sliwowski Z, Pajdo R, Duda A, Ptak A and Hahn EG. (2003). Implications of reactive oxygen species and cytokines in gastroprotection against stress-induced gastric damage by nitric oxide releasing aspirin. International Journal of Colorectal Disease. 18:320-329.
[https://doi.org/10.1007/s00384-002-0451-2]

-
Cerecetto H, Gerpe A, González M, Aran VJ and de Ocariz CO. (2005). Pharmacological properties of indazole derivatives: Recent developments. Mini-Reviews in Medicinal Chemistry. 5:869-878.
[https://doi.org/10.2174/138955705774329564]

-
Gaikwad DD, Chapolikar AD, Devkate CG, Warad KD, Tayade AP, Pawar RP and Domb AJ. (2015). Synthesis of indazole motifs and their medicinal importance: An overview. European Journal of Medicinal Chemistry. 90:707-731.
[https://doi.org/10.1016/j.ejmech.2014.11.029]

- Guzik TJ, Korbut R and Adamek-Guzik T. (2003). Nitric oxide and superoxide in inflammation. Journal of Physiology and Pharmacology. 54:469-487.
-
Huang G, Mao J, Ji Z and Ailati A. (2015). Stachyose-induced apoptosis of caco-2 cells via the caspase-dependent mitochondrial pathway. Food and Function. 6.3:765-771.
[https://doi.org/10.1039/C4FO01017E]

-
Im AR, Kim YH, Lee HW and Song KH. (2016). Water extract of Dolichos lablab attenuates hepatic lipid accumulation in a cellular nonalcoholic fatty liver disease model. Journal of Medicinal Food. 19:495-503.
[https://doi.org/10.1089/jmf.2015.3623]

- Kante K and Reddy CS. (2013). Anti diabetic activity of Dolichos lablab(seeds) in streptozotocin-nicotinamide induced diabetic rats. Hygeia Journal for Drugs and Medicines. 5:32-40.
-
Michiels C, Raes M, Toussaint O and Remacle J. (1994). Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free radical Biology and Medicine. 17:235-248.
[https://doi.org/10.1016/0891-5849(94)90079-5]

-
Nishizawa-Yokoi A, Yabuta Y and Shigeoka S. (2008). The contribution of carbohydrates including raffinose family oligosaccharides and sugar alcohol to protection of plant cells from oxidative damage. Plant Signaling and Behavior. 3:1016-1018.
[https://doi.org/10.4161/psb.6738]

-
Niu XF, Smith CW and Kubes P. (1994). Intracellular oxidative stress induced by nitric oxide synthesis inhibition increases endothelial cell adhesion to neutrophils. Circulation Research. 74:1133-1140.
[https://doi.org/10.1161/01.RES.74.6.1133]

-
Osman MA. (2007). Effect of different processing methods, on nutrient composition, antinutrional factors, and in vitro protein digestibility of Dolichos lablab bean[Lablab purpuresus (L) Sweet]. Pakistan Journal of Nutrition. 6:299-303.
[https://doi.org/10.3923/pjn.2007.299.303]

- Peterbauer T and Richter A. (2001). Biochemistry and physiology of raffinose family oligosaccharides and galactosyl cyclitols in seeds. Seed Science Research. 11:185-197.
- Priya S and Jenifer S. (2014). Antibacterial activity of leaf and flower extract of Lablab purpureus against clinical isolates of Staphylococcus aureus. Research and Reviews: A Journal of Drug Design and Discovery. 1:5-7.
-
Rahman A, Malik S, Hasan SS, Choudhary MI, Ni CZ and Clardy J. (1995). Nigellidine: A new indazole alkaloid from the seeds of Nigella sativa. Tetrahedron Letters. 36:1993-1996.
[https://doi.org/10.1016/0040-4039(95)00210-4]

-
Ramakrishna V, Jhansi Rani P and Pao RR. (2007). Hypocholesterolemic effect of diet supplemented with Indian bean (Dolichos lablab L. var lignosus) seeds. Nutrition and Food Science. 37:452-456.
[https://doi.org/10.1108/00346650710838117]

-
Salvemini D, Ischiropoulos H and Cuzzocrea S. (2003). Roles of nitric oxide and superoxide in inflammation. In Winyard PG and Willoughby DA (ed.). In Inflammation Protocols. Methods in Molecular Biology. vol 225. Humana Press. Totowa, NJ, USA. p.291-303.
[https://doi.org/10.1385/1-59259-374-7:291]

-
Stoyanova S, Geuns J, Hideg E and Van Den Ende W. (2011). The food additives inulin and stevioside counteract oxidative stress. International Journal of Food Sciences and Nutrition. 62:207-214.
[https://doi.org/10.3109/09637486.2010.523416]

-
Takeuchi A, Hori M, Sato S, Ban HS, Kuchimaru T, Kizaka-Kondoh S, Yamori T and Nakamura H. (2012). Synthesis and biological activity of furanylindazoles as inhibitors of hypoxia inducible factor(HIF)-1 transcriptional activity. Medicinal Chemistry. Communication. 3:1455-1461.
[https://doi.org/10.1039/c2md20134h]

-
Tiidus PM. (1998). Radical species in inflammation and overtraining. Canadian Journal of Physiology and Pharmacology. 76:533-538.
[https://doi.org/10.1139/y98-047]

-
Warleta F, Quesada CS, Campos M, Allouche Y, Beltrán G and Gaforio JJ. (2011). Hydroxytyrosol protects against oxidative DNA damage in human breast cells. Nutrients. 3:839-857.
[https://doi.org/10.3390/nu3100839]

-
Ye XY, Wang HX and Ng TB. (2000). Dolichin, a new chitinase-like antifungal protein isolated from field beans(Dolichos lablab). Biochemical and Biophysical Research Communications. 269:155-159.
[https://doi.org/10.1006/bbrc.2000.2115]