Antimicrobial Activity against Food-hazardous Microorganisms, Dermatophytes, and Pytopathogens and Antioxidative Activity of Sancho Oil
 ; Seung Mi Kang2
; Seung Mi Kang2 ; Seong Hyeon Yong3
; Seong Hyeon Yong3 ; Yu Won Seol4
; Yu Won Seol4 ; Eun Ji Choi5
; Eun Ji Choi5 ; Jun Ho Park6
; Jun Ho Park6 ; Chan Yeol Yu7
; Chan Yeol Yu7 ; Tamirat Solomon8
; Tamirat Solomon8 ; Myung Suk Choi9, †
; Myung Suk Choi9, †
This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Although Sancho (Zanthoxylum schinifolium Siebold & Zucc) oil has traditionally been used for its antibiotics properties, there is currently a lack of scientific evidence regarding its biological activities. In this study, we investigated the antimicrobial and antioxidant activities of Sancho oil against food-hazardous microorganisms, phytopathogens, and dermatophytes.
We investiated the antimicrobial activity of Sancho oil against 11 food-hazardous microorganisms, nine phytopathogens, and six dermatophytes. The Sancho oil was found to show the strongest antibacterial activity against Shigella flexneri and Listeria spp. Sancho oil also showed high antifungal activity against plant pathogens, particularly Fusarium oxysporum, and showed antimicrobial activity against dermatophytes such as Trichophyton rubrum, Microsporum canis and Candida albicans. The antioxidant activity of Sancho oil was measured using the DPPH method, and was found to be stronger than that of unrefined oil. Moreover, this activity increased with increasing oil concentration.
We found that Sancho oil showed differing antimicrobial activities against food-hazardous microorganisms, dermatophytes, and plant pathogens. The antimicrobial activity spectrum of Sancho oil was not broad and varied among microbial strains. On the basis of our findings, we consider that Sancho oil could be used an antibacterial material for food-borne S. flexneri and Listeria spp., a biopesticide for Fusarium spp., and a treatment for dermatophytes such as T. rubrum.
Keywords:
Sancho Oil, Food-hazardous Microorganisms, Dermatophytes, Phytopathogenic MicroorganismsINTRODUCTION
Sancho (Zanthoxylum schinifolium Siebold & Zucc) is a deciduous shrub belonging to the Rutaceae. It grows in the mid-mountain and valley and is 3 - 5 m high. Young leaves and berries are used as spices because of their unique fragrance and acidity, and have long been used in the private sector as traditional spices and medicinal plants (Lee et al., 2003). Sancho oil contains limonene, citronellal, phellandrene, sanshool, and flavonoid (Kim et al., 2000). It has been widely used as an anti-inflammatory, diuretic, detoxifying, antiparasitic, anal pain, and dry place. It stimulates the stomach to improve metabolism, helps digestion and stops diarrhea (Kim et al., 2000). Seo et al. (2012) also found that oral administration of 200 ㎎/㎏/day Z. schinifolium seed oil for 13 weeks improved most immune-related cells and was biologically safe. Z. schinifolium oil has been reported to exhibit pesticidal toxicity against Dermatophagoides farinae, D. pteronyssinus and Tyrophagus putrescentiae, and aphids (Lee, 2016).
On the other hand, the plant field has been extensively studied in materials having antioxidant and antimicrobial effects during the development of new materials (Goñi et al., 2009). In particular, various side effects due to allergies have been reported in the case of long-term administration of a chemical synthetic preparation (Lee and Park, 2011). As an alternative, many studies have been conducted on herbal medicines such as plants and traditional medicines.
Sancho oil is very promising antimicrobials and are expected to be developed as a promising species (Lee et al., 2012). Diao et al. (2013) reported that essential oils from Z. schinifolium has antimicrobial activity against Staphylococcus epidermidis and antiviral activity against food-borne viral surrogates, feline calicivirus-F9 (FCV-F9). However, most of the research has been conducted on the biological activities of Z. schinifolium essential oils and extracts extracted from stems and fruits with organic solvents (Kim et al., 2000). It is also widely used in Z. piperitum, a similar species of Z schinifolium. Therefore, research on Z. schinifolium seed oil, Sancho oil, is rare.
In this study, we investigated the antimicrobial and antioxidant activities of various microorganisms such as food-hazardous microorganisms, dermatophytes, and plant pathogens.
MATERIALS AND METHODS
1. Materials
The Sancho (Zanthoxylum schinifolium Siebold & Zucc) oil used for the antimicrobial test according to acidity was collected from Hadong J. Farm (Hadong, Korea) on November 25, 2012, and Sancho oil stored for 1 year was used high acidity Sancho oil (HA1), low acidity Sancho oil (LA1) was harvested in November 2013 and examined for its antibacterial activity. Also, all reagents used in the experiment were used as extra pure grade.
The microorganisms used in this study were 11 food-hazardous microorganisms, 6 dermatophytes, and 9 plant pathogens (Table 1). Microbial strains were purchased from KCTC (Korean Collection for Type Cultures, Jeongup, Korea), KCCM (Korean Culture Center of Microorganisms, Seoul, Korea) and KACC (Korean Agricultural Culture Collection, Wanju, Korea). The microorganisms were cultured by inoculating 100 ㎖ of the microbial stock suspension in 5 ㎖ nutrient salt (Difco Laboratories Inc., Detroit, MI, USA) in a shaking incubator at 37℃ for 24 hours under aerobic conditions.
2. Antimicrobial activity of Sancho oil against food-hazardous bacteria
Antimicrobial activity assay was determined by the disk agar plate diffusion method. Each test strain was incubated in the Tiyptic soy (TS) agar liquid medium for 24 hours, and the plate medium was prepared by coagulating 20 ㎖ of 1.5% agar sterilized in each growth medium in petridish and adding 0.1 ㎖ of each test microorganism. The glass rods were applied to spread evenly on the medium. A paper disc (8 ㎜, Toyo Roshi Kaicha Ltd., Tokyo, Japan) in which Sancho oil was injected at a certain concentration was adsorbed onto a plate medium, and 30 ㎕ of sterilized water was injected. The antimicrobial effect was compared by measuring the diameter (㎜) of inhibition clear zone.
The antibacterial activity according to the acidity of Sancho oil was carried out by the method mentioned above.
3. Antifungal activity test of Sancho oil against phytopathogens
The antifungal activity test of phytopathogens of Sancho oil was done with a slight modification of the method of Mohana and Raveesha (2007). 50 ㎖ potato dextrose agar (PDA) liquid medium was added to a 250 ㎖ Erlenmeyer conical flask, and Sancho oil dissolved in 20 ㎎/㎖ dimethyl sulfoxide (DMSO) was added. The flasks were inoculated with 5 ㎜ diameter mycelia disc of phytopathogens taken from 7 days old culture and incubated for 7 days at 22 ± 1℃ under alternate cycles 12 hours. light and 12 hours. darkness. After incubation the content of the each flask was poured into a preweighed Whatman No. 1 filter paper. The filter paper with the mycelial mat was dried in an oven at 60℃ until a constant weight was reached. The dry weight of the mycelia was determined by subtracting the weight of the filter paper from the total weight of the filter paper with mycelia. Three replicates were maintained for each treatment. The percent inhibition of mycelial growth was calculated using the formula:
Percent inhibition = C - [T/C × 100]
where C = Mycelial weight in control, T = Mycelial weight in treatment.
The antimicrobial activity test according to the degree of acidity of Sancho oil was carried out as described above.
4. Antifungal activity test of Sancho oil against dermatophytes
Sancho oil was added to sabourud dextrose agar (SDA, Merck KGaA, Darmstadt, Germany) medium and Sancho oil dissolved in 20 ㎎/㎖ DMSO. Then, 5 ㎜ diameter dermatophytes discs were inoculated for 7 days and incubated for 12 hours at 22 ± 1℃ for 7 days. The culture conditions were performed in the same manner as the phytopathogens mentioned above. The contents of each flask were pre-weighed by filter paper (Whatman No. 1, Whatman Inc., Florham Park, NJ, USA) poured into filter paper. The filter paper containing mycelium was dried in an oven at 60℃ until a constant weight was reached. The dry weight of the mycelium was measured by subtracting the weight of the filter paper from the total weight of the filter paper using the mycelium. Three replicates were maintained for each treatment. Mycelial growth inhibition was calculated using the same formula as phytopathogens.
5. Measurement of antioxidative activity by DPPH method
In this experiment, a Sancho oil sample (90 - 500 ㎎/ℓ) and the solution of 0.4 mM 1,1-diphenyl-2-picrylhydrazyl (DPPH) dissolved in ethanol were taken and mixed well. After incubation at 20℃ for 20 minutes, absorbance was measured at 517 ㎚. Electron donating ability (EDA, %) was measured by the difference of the absorbance of the sample added and blank sample as a percentage (%).
The antioxidant activity of the purified Sancho oil was investigated. First, refining of Sancho oil was carried out by the method of Kang et al. (2017). In Sancho oil purification, Sancho oil was first slowly stirred at a very low speed in a water bath using phosphoric acid and acetic acid at 0.5, 1.0 and 2.0% concentration. The mixture was heated at 40℃ - 50℃ and maintained at a final temperature of 85℃ for 15 - 20 minutes. The mixture was allowed to stand for 20 minutes and the precipitate was removed. The mixture was centrifuged at 2,000 rpm for 30 minutes to remove degumming oil. The antioxidative activity of DPPH method was measured by using the above-prepared Sancho oil and sesame oil.
6. Statistical analysis
All experiments were performed 3 times. Data are presented as means and standard deviation and analyzed by One-way ANOVA using the IBM SPSS statistical package (Ver. 24, IBM Co., Armonk, NY, USA). Means were compared at 5% significance level using Duncan’s Multiple Range Test (DMRT) comparison (p < 0.05).
RESULTS
1. Antimicrobial activity against food-hazardous bacteria
The antimicrobial activity of Sancho oil against food-hazardous bacteria is shown in Table 2. The antimicrobial activity of Sancho oil against food-hazardous bacteria was different for each microbial strain. Sancho oil showed strong antimicrobial activity against S. flexneri among tested food-hazardous bacteria. In addition, Sancho oil showed also strong antimicrobial activity against L. monocytogenes and E. coil.
The antimicrobial activity of food-hazardous bacteria was different according to the degree of rancidity of Sancho oil (Table 2). LA1 Sancho oil had lower antibacterial activity than HA1 Sancho oil with higher acid value.
2. Antifungal activity against phytopathogens
The antifungal activity of Sancho oil against phytopathogens was investigated (Table 3). Antifungal activity against phytopathogens varied with treatment concentrations. As the concentration of Sancho oil increased, antifungal activity increased in most phytopathogens strains. The antifungal activity of low concentration Sancho oil of less than 3% was highest in F. oxysporum, followed by P. roqueforti. F. oxysporum was the strongest at 5% treatment, followed by A. awamori, A. niger, P. roqueforti, and A. flavus var. flavus.
The antifungal activity of phytopathogens according to the acidity of Sancho was measured (Fig. 1). The antifungal activities of phytopathogens except Rhizopus spp. showed higher antifungal activity in Sancho oil, which has lower acidity than that of high acidity Sancho oil. The antifungal activity of low concentration Sancho oil of less than 3% was highest in F. oxysporum, followed by P. roqueforti. F. oxysporum was the strongest at 5% treatment. A. awamori increased sharply from 3% and showed 98% antifungal activity at 6%. The antifungal activity of A. niger and A. flavus was weaker than A. awamori, but showed the same tendency.
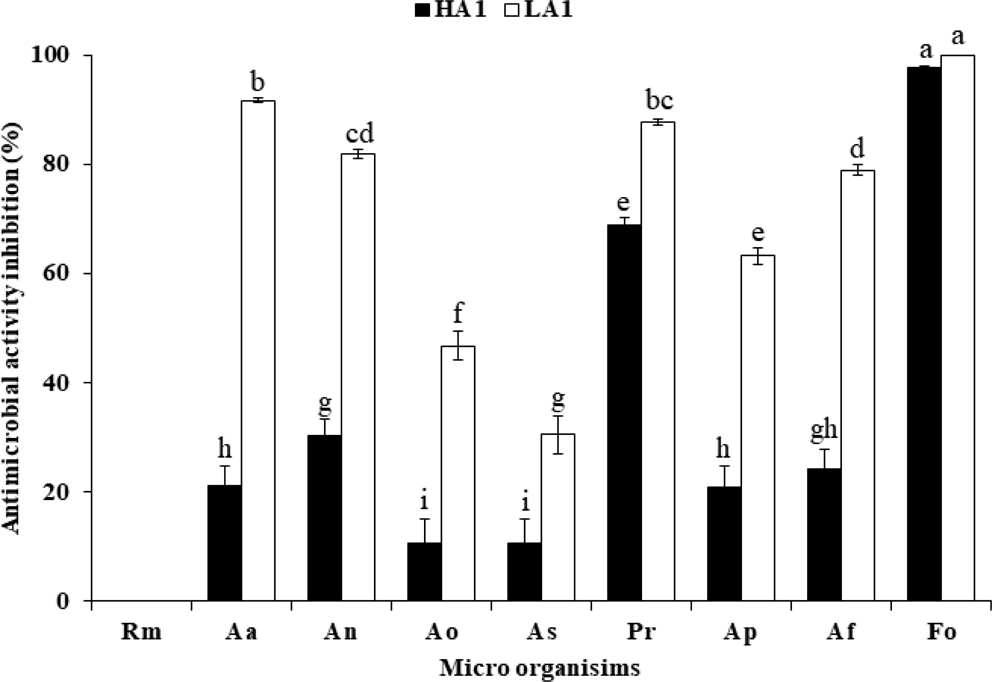
Antimicrobial activity of Sancho oil against plant pathogenic fungi according to acidity.Rm; Rhizopus microsporus var. oligosporus, Aa; Aspergillus awamori, An; Aspergillus niger, Ao; Aspergillus oryzae, As; Aspergillus sojae, Pr; Penicillium roqueforti, Ap; Aspergillus parasiticus, Af; Aspergillus flavus var. flavus, Fo; Fusarium oxysporum.
3. Antimicrobial activity against dermatophytes
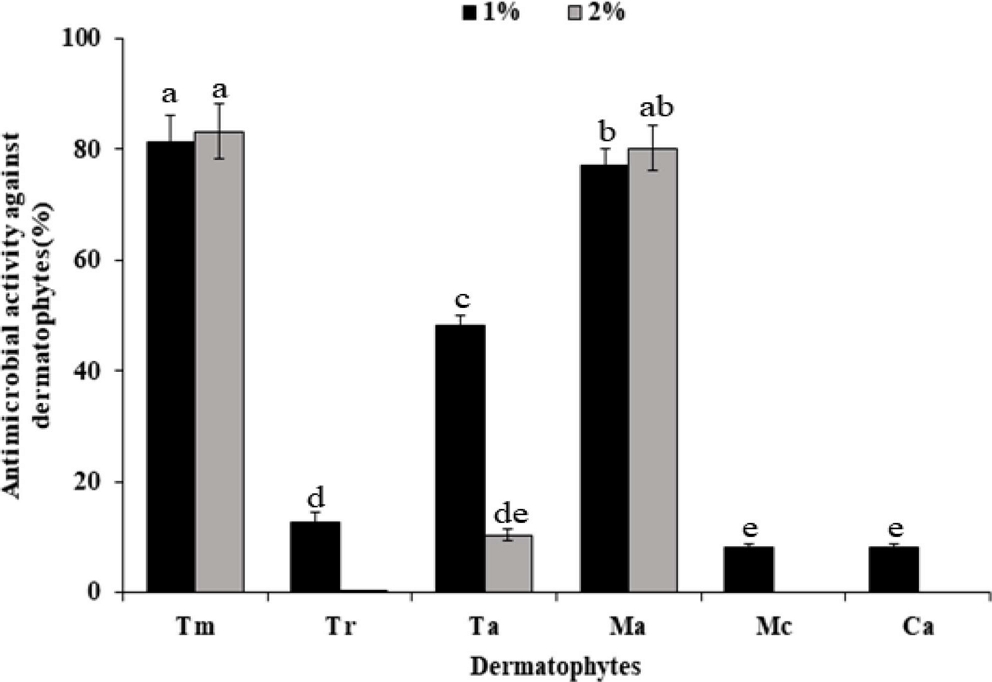
The antimicrobial activity of Sancho oil against six dermatophytes was investigated (Fig. 2). In the growth of dermatophytes, the higher the concentration of Sancho oil, the more the growth of the dermatophytes was inhibited than the control group. Among them, Sancho oil showed strong antimicrobial activity against M. canis and C. albicans. T. rubrum, M. canis, and C. albicans did not grow when treated with more than 4% of Sancho oil. However, Sancho oil had low antimicrobial activity against T. mentagrophytes and M. audouinii.
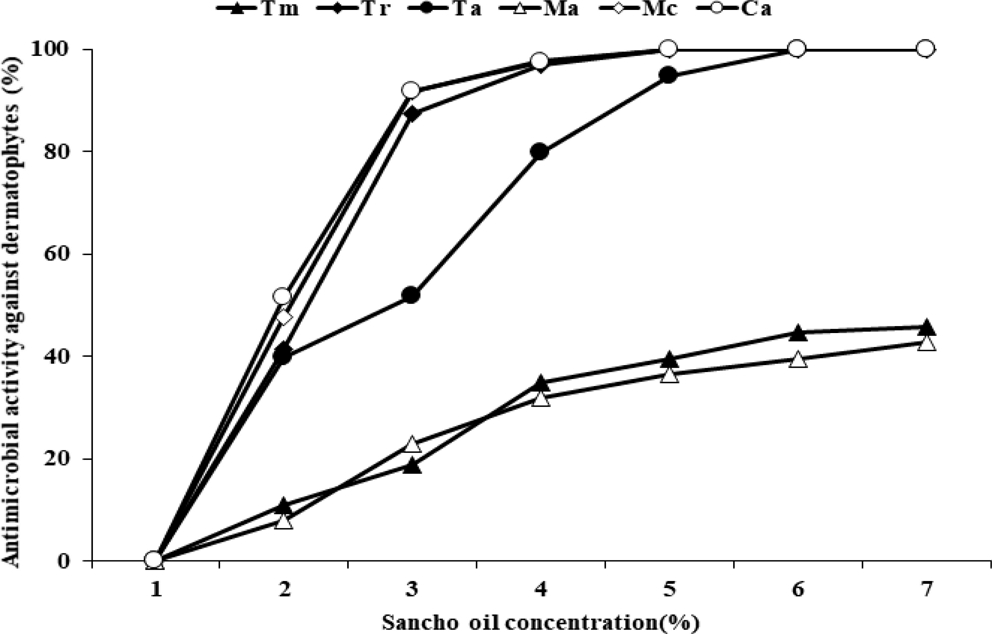
Antibacterial activity against dermatophytes according to the concentration of Sancho oil.Tm; Trichophyton mentagrophytes, Tr; Trichophyton rubrum, Ta; Trichophyton ajelloi, Ma; Microsporum audouinii, Mc; Microsporum canis, Ca; Candida albicans.
The antimicrobial activity of Sancho oil against dermatophytes was different according to treatment period (Table 4). Except for T. mentagrophytes and M. audouinii, it strongly inhibited the growth of dermatophytes from the 2nd day of cultivation. After 4 days, the growth was completely inhibited.
The effect of antimicrobial activity of dermatophytes was shown to be high when Sancho oil with low acid value was used (Fig. 3). However, the growth of T. mentagrophytes and M. audouinii was lower than that of the control group, but there was no decrease in the acidity of Sancho oil.
4. Antioxidant activity test of Sancho oil
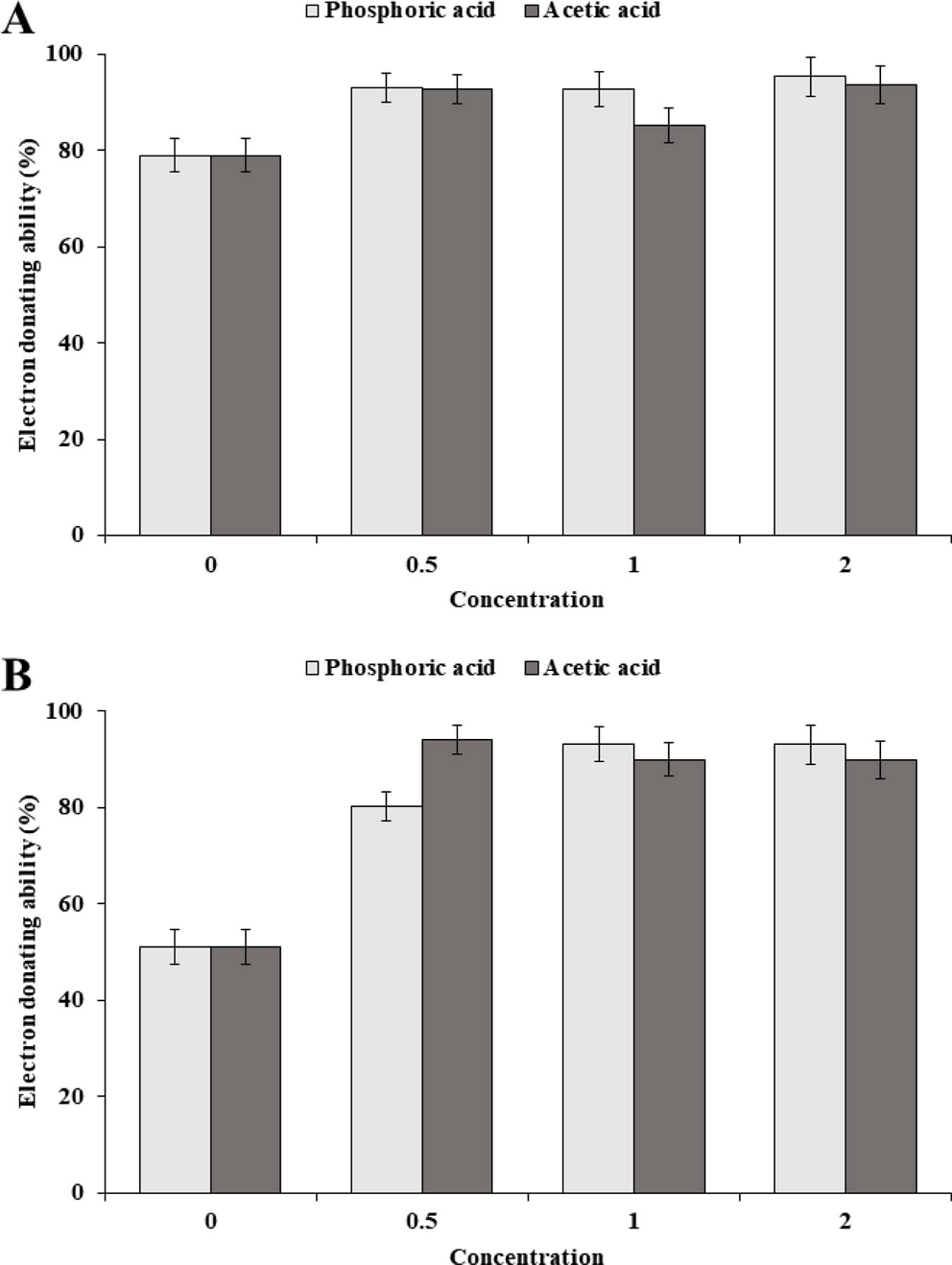
The electron donating ability (EDA, %) values of Sancho oil varied slightly depending on the acidity and concentration of oil (Fig. 4). The EDA value of Sancho oil increased with increasing oil concentration. In the case of HA1, the EDA value gradually increased according to the concentration, while the EDA value rapidly increased in the LA1 up to 90 ㎎/ℓ, but did not increase significantly at the higher concentration.
Purification yield of phosphate and acetic acid was higher in phosphate treatment than acetic acid treatment (data not shown). Purification yields for these two treatments were 84% - 92%.
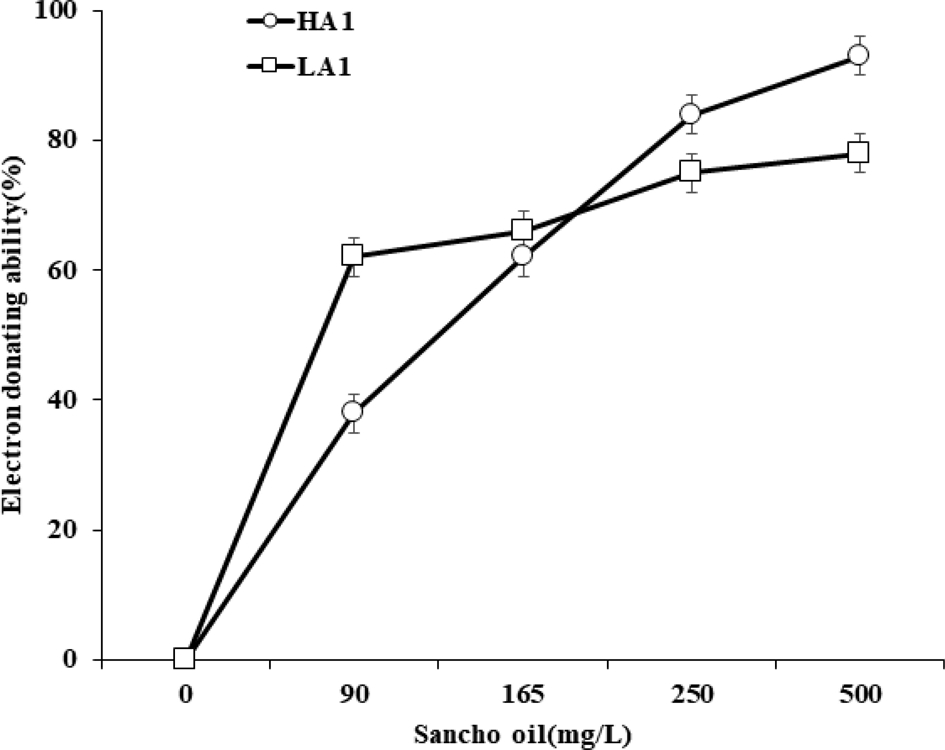
The free radical scavenging effect of using DPPH method of different Sancho oil.HA1; high acidity Sancho oil, LA1; low acidity Sancho oil.
The EDA showed the difference of antioxidant ability of Sancho oil purified by phosphoric acid and acetic acid treatment (Fig. 5). The antioxidant ability of oil purified by phosphoric acid treatment was slightly improved compared with that of acetic acid treated oil. The antioxidant ability was slightly increased as the concentration of the two refining reagents was increased. However, there was no significant difference in antioxidant capacity between high and low oil.
DISCUSSION
The antimicrobial activity of Sancho oil against food-hazardous bacteria differed from microorganism to microorganism, among which it was the strongest against L. monocytogenes. Lee et al. (2012) examined the antimicrobial activity of food-hazardous bacteria such as L. monocytogenes, S. aureus, and Vibrio parahaemolyticus. The antimicrobial activity of Sancho oil appears to be due to the essential oil component contained in a small amount in the Sancho oil. There are many reports on the antibacterial activity of plant essential oils. The essential oil components of the fruit of Z. schinifolium were linalool (28.2%), limonene (13.2%) and sabinene (12.1%) and showed strong antimicrobial activity against food-hazardous pathogens (Diao et al., 2013).
Sancho oil, which had a high acid value, showed stronger antibacterial activity against food-hazardous bacteria than oil with low acid value. The rancidity of Sancho oil reacts with oxygen by the contact of heat, air, water, etc., and hydroperoxide is produced (Chu and Luo, 1994). In addition, it is considered that the change of unsaturated fatty acid was caused by natural oxidation of Sancho oil. Chun and Kim (1992) reported that oleic acid, linoleic acid, pentanal, and hexanal are produced when edible oils are oxidized. Yoon et al. (1988) reported that automatic oxidation of soybean oil reduces unsaturated fatty acids such as linolenic acid, linoleic acid, oleic acid and increases fatty acids such as palmitic acid.
The growth of microorganisms is influenced by the content of acidic substances and the incubation temperature, and it is known that it can be controlled by controlling the pH (Lund et al., 2014). Lowered acidity appears to have inhibited microbial growth. Oxidation indicates the number of carbon atoms is between 7 - 13 fatty acids with strong antibacterial action, known to represent a double bond, the material is more strong antifungal action in the these fatty acid carbon (Peck and Russ, 1947).
Sancho oil showed antifungal activity against various phytopathogens. Sancho oil has a very strong antifungal activity of F. oxysporum. F. oxysporum is a pathogenic fungus commonly found around the world. It is a soil borne ascomycete causing Fusarium wilt, on many economically important crops. This pathogen comprises of over 120 known strains and each of which is specific to unique host plant in which it causes disease (Agrios, 2005).
Assays of plant maintenance against fungi are known to be very difficult. More methods have been tried to monitor the growth rate of fungi, and the measurement of the hyphae dry weight or increased colony diameter obtained in liquid culture. In this study, the antifungal activity of Sancho oil was assayed using the agar plate-dry weight method. The agar plate-dry weight method is considered a very good method as a tool for assessing mycelial growth of vegetable oil etc.
Antimicrobial activity of phytopathogens of Sancho oil appears to be due to the fatty acids contained. Plant pathogens such as fungi, bacteria, nematodes and viruses can damage plant growth and biomass production. Harvest losses from plant diseases worldwide are known to be more than 35% in developing countries (Arici and Sanli, 2014). It occurs in the whole growing season, initially the leaves are bent and yellowed, and sometimes necrotic symptoms at the tip of the leaves progress downward. If the disease progresses severely, the whole leaf will wither and dry white. Among these pathogens, fungi and bacteria are the main pathogens and cause many diseases in plants. Most of the plants are economically reduced when they are infected with Rhizoctonia solani, Pythium ultimum, Botrytis cinerea, Pyhtopthora capsici, and Erwinia caratovora (Arici et al., 2011).
Many pest control agents are very dangerous to the environment and soil, as well as methyl bromide, a pest control agent commonly used in high-value crops (Kizil et al., 2005). Traditional medicine is an important source of potentially useful compounds for the development of chemotherapy agents. Various biomaterials, including plants, can be used to find effective alternatives to synthetic drugs (Gaikwadi et al., 2003).
Sancho oil showed different antimicrobial activity in 6 dermatophytes. Among them, M. canis and C. albicans were the most resistant microorganisms against dermatophytes. Millions of people around the world are affected by superficial fungal infections, the most common dermatophytes. This infection, which occurs in both healthy and immunocompromised persons, is mainly caused by dermatophytes. Trichophyton spp. dermatophytes such as M. canis are generally involved in such infections (Chuang et al., 2007).
They cause common infections in humans, are difficult to control effectively, and drug development is still weak. Plant-derived natural products inhibit pathogen growth without harming the host (Bokhari, 2009). Plant-derived essential oils and extracts are regarded as non-toxic compounds and have antimicrobial and anti-dermatological properties (Ibrahim and El-Salam, 2015). They have been shown to inhibit fungal pathogens (Chuang et al., 2007; Ibrahim and El-Salam, 2015).
Sancho oil showed antimicrobial activity against T. mentagrophytes and M. audouinii species. This means that Sancho oil is applied to industries such as cosmetics as natural materials.
Sancho oil showed slightly more antioxidant activity after purification. Crude vegetable oils and fats contain many undesirable substances such as free fatty acids, pigments, gums, waxes, phosphates, trace metals, pesticide residues and odorous substances, which must be removed to produce a smooth, pleasant and stable product taste (Ghazani et al., 2013). Most of these have an undesirable effect on the odor, odor, appearance and shelf life of the oil and must therefore be removed from the vegetable oil by a chemical or physical purification process (Verleyen et al., 2002).
Sancho oil showed different antioxidant activities in different regions of production. The antioxidative activities of Sancho oil were similar or slightly lower than those of red pepper seed, pumpkin seed and camellia oil (Ku and Lee, 2018).
Antioxidant activity seems to be due to the fatty acids in the Sancho oil. The fatty acids that constitute the largest proportion of the fatty acids of Sancho oil are oleic acid, accounting for about 30% of total fatty acids, followed by linoleic acid and linolenic acid, accounting for 27% and 20%, respectively (Kim et al., 2012). It is known to contain excellent oleic acid and palmitic acid (9% - 12%) and linoleic acid (1% - 3%), which are 85% - 90% of camellia oil and have excellent antioxidative activity (Ku and Lee, 2018).
As described above, Sancho oil showed different antimicrobial activity for each strains of food-hazardous microorganism, phytopathogenic and dermatophytes microorganism, and showed a relatively strong antimicrobial activity according to strains. While the antimicrobial activity of Sancho oil is not as strong as the solvent extracts from the leaves of the herb, it has a variety of physiological activities, which could be utilized in future cosmetics and dietary supplements.
Acknowledgments
This work was carried out with support of Forest Science and Technology Project (S111616L170110) Korea Forest Service, Republic of Korea.
References
-
Agrios GN. (2005). Plant pathology. 5th ed. Elsevier Academic Press. Amsterdam, Nederland. p.105-123.
[https://doi.org/10.1016/B978-0-08-047378-9.50009-9]

-
Arici SE and Sanli A. (2014). Effect of some essential oils against Rhizoctonia solani and Streptomycetes scabies on potato plants in field conditions. Annual Research and Review in Biology. 4:2027-2036.
[https://doi.org/10.9734/ARRB/2014/8526]

- Arici ȘE, Özgönen H, Șanlı A, Polat M and Yasan G. (2011). Antimicrobial activity of essential oils against agricultural plant pathogenic fungi and bacteria. The French Association of Plant Protection(AFPP), Fourth International Conference on non Chemical Crop Protection Methods. https://www.cabdirect.org/cabdirect/abstract/20113378869, . (cited by 2019 Dec 13).
- Bokhari FM. (2009). Antifungal activity of some medicinal plants used in Jeddah. Mycopath. Jeddah, Saudi Arabia. 7:51-57.
-
Chu YH and Luo S. (1994). Effects of sugar, salt and water on soybean oil quality during deep-frying. Journal of the American Oil Chemists' Society. 71:897-900.
[https://doi.org/10.1007/BF02540470]

-
Chuang PH, Lee CW, Chou JY, Murugan M, Shieh BJ and Chen HM. (2007). Anti-fungal activity of crude extracts and essential oil of Moringa oleifera Lam. Bioresource Technology. 98:232-236.
[https://doi.org/10.1016/j.biortech.2005.11.003]

- Chun HN and Kim ZU. (1992). Evaluation of vegetable oil rancidity by headspace gas chromatographic analysis. Journal of the Korean Agricultural Chemical Society. 35:36-41.
-
Diao WR, Hu QP, Feng SS, Li WQ and Xu JG. (2013). Chemical composition and antibacterial activity of the essential oil from green huajiao(Zanthoxylum schinifolium) against selected foodborne pathogens. Journal of Agricultural and Food Chemistry. 61:6044-6049.
[https://doi.org/10.1021/jf4007856]

- Gaikwadi SS, Vadlamudi VP, Waghmaee SP, Maral VJ, Ranteke VD and Dhok AP. (2003). Phytochemical analysis of aqueous extract of few medicinal plants. PKV Research Journal. 27:91-92.
-
Ghazani SM, García-Llatas G and Marangoni AG. (2013). Minor constituents in canola oil processed by traditional and minimal refining methods. Journal of the American Oil Chemists' Society. 90:743-756.
[https://doi.org/10.1007/s11746-013-2215-2]

-
Goñi P, López P, Sánchez C, Gómez-Lus R, Becerril R and Nerín C. (2009). Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chemistry. 116:982-989.
[https://doi.org/10.1016/j.foodchem.2009.03.058]

-
Ibrahim SY and El-Salam AMM. (2015). Anti-dermatophyte efficacy and environmental safety of some essential oils commercial and in vitro extracted pure and combined against four keratinophilic pathogenic fungi. Environmental Health and Preventive Medicine. 20:279-286.
[https://doi.org/10.1007/s12199-015-0462-6]

- Kang SM, Kim HG, Yang WH, Yong SH, Park DJ, Park JH, Enukwa EH and Choi MS. (2017). Changes in the Physicochemical characteristics of Sancho oil according to the purification process. Korean Journal of Medicinal Crop Science. 25:296-304.
- Kim J, Cho YS, Seo KI, Joo OS and Shim KH. (2000). Antimicrobial activities of Zanthoxylum schinifolium and Zanthoxylum piperitum leaves. Journal of Korean Society of Postharvest Science and Technology. 7:195-200.
- Kim KG, Song HJ, Jeong MJ, Seo YL Kim DI, Lim JT, Kim HK, Kang SM, Kim HJ and Choi MS. (2012). Fatty acid content and physicochemical characteristics of Sancho (Zanthoxylum piperitum DC.) seed oil. Journal of Forest Genetics and Physiology. 1:1-7.
- Kim YD, Kang SK, Choi OJ, Lee HC, Jang MJ and Shin SC. (2000). Screening of antimicrobial activitiy of Chopi (Zanthoxylum piperitum A.P. DC.) extract. Journal of Korean Society of Food Science and Nutrition. 29:1116-1122.
-
Kizil S, Uyar F and Sagir A. (2005). Antibacterial activities of some essential oils against plant pathogens. Asian Journal of Plant Sciences. 4:225-228.
[https://doi.org/10.3923/ajps.2005.225.228]

- Ku HY and Lee KY. (2018). Evaluation of the antioxidant effects of extracted seed oils by pressure method using domestic seeds and nuts. Journal of the Korea Academia Industrial cooperation Society. 19:655-661.
- Lee CJ, kim MS, Shen JY, Kim YD and Shin JH. (2003). The extraction condition of pungent compounds from Zanthoxylum piperitum D.C pericarps by using supercritical fluid extraction. Korean Journal of Medicinal Crop Science. 11:19-23.
-
Lee HS. (2016). Insecticidal toxicities and essential oil compositions of Zanthoxylum piperitum and Zanthoxylum schinifolium fruits in Korea. Journal of Essential Oil Bearing Plants. 19:2065-2071.
[https://doi.org/10.1080/0972060X.2016.1249415]

-
Lee JH, Jang M, Seo J and Kim GH. (2012). Antibacterial effects of natural volatile essential oil from Zanthoxylum piperitum A.P. DC. against foodborne pathogens. Journal of Food Biochemistry. 36:667-674.
[https://doi.org/10.1111/j.1745-4514.2011.00581.x]

- Lee SG and Park CI. (2011). A study on the anti-microbial effect on S. mutans and anti-oxidant effect of Zanthoxylum pericarpium extract. Korean Journal of Herbology. 26:181-185.
-
Lund P, Tramonti A and Biase DD. (2014). Coping with low pH: Molecular strategies in neutralophilic bacteria. FEMS Microbiology Reviews. 38:1091-1125.
[https://doi.org/10.1111/1574-6976.12076]

- Mohana DC and Raveesha KA. (2007). Anti-fungal evaluation of some plant extracts against some plant pathogenic field and storage fungi. Journal of Agricultural Technology. 4:119-137.
-
Peck SM and Russ WR. (1947). Propionate-caprylate mixtures in the treatment of dermatomycoses. Archives Dermatology Syphilology. 56:601-613.
[https://doi.org/10.1001/archderm.1947.01520110047007]

-
Seo JW, Park DH, Li YC, Kang HJ, Xu HD, Kim YJ, Lee JH, Lee MS, Lee IC, Lee YL, Ahn JB, Cho SC and Lee MJ. (2012). The Zanthoxylum schinifolium seed oil modulates immune function under the biological safety level. Molecular Cell Toxicolology. 8:179-185.
[https://doi.org/10.1007/s13273-012-0022-8]

-
Verleyen T, Sosinska U, Ioannidou S, Verhe R, Dewettinck K, Huyghebaert A and de Greyt W. (2002). Influence of the vegetable oil refining process on free and esterified sterols. Journal of the American Oil Chemists' Society. 79:947-953.
[https://doi.org/10.1007/s11746-002-0585-4]

- Yoon SH and Kim JW. (1988). Antioxidative effects of various antioxidants on the soybean oil. Journal of Korean Society of Food Science and Nutrition. 17:19-23.