Ginsenoside Analysis of Panax ginseng C. A. Meyer Culture Broth in a Bioreactor and Its Application in Inducing Biological Changes in Leafy Vegetables
This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The aim of this study was done to identify whether mass produced wild ginseng culture broth prepared from cultivated wild ginseng roots could have an application in enhancing the agricultural utility value of leafy vegetables.
Leafy vegetables Lactuca sativa and Brassica juncea were treated with wild ginseng culture broth. Plants were examined and treatment (100 ㎖) applied twice a week over an eight week period. Total phenolic and flavonoid content of treated plants was then measured. Wild ginseng culture broth treatment resulted in phenolic and flavonoid content of 0.40 ㎎·GAE/㎖ and 0.36 ㎎·QE/㎖, respectively in L. sativa. When treated with wild ginseng culture broth, free radical scavenging ability was found to be higher in both L. sativa and B. juncea whereas antimicrobial activity was found to be higher in B. juncea (625 ㎍/㎖) than in L. sativa. Inorganic element analysis of L. sativa and B. juncea showed that Ca and Mg were higher in the wild ginseng broth treatment group, whereas harmful elements such as As were reduced.
Rather than discarding the wild ginseng culture broth, it can be used as a fresh biomaterial by reprocessing it as agricultural products that can promote growth and improve functionality in plants.
Keywords:
Panax ginseng C. A. Meyer, Leafy Vegetables, Biological Activities, General Components, Antimicrobial Activity, Ginseng Culture BrothINTRODUCTION
Panax ginseng C. A. Meyer (Family Araliaceae) is a perennial herb known for its medicinal qualities; it tastes sweet and bitter, is considered warm and refreshing to consume, and is reputed to have both therapeutic and pharmacological benefits (Yoo et al., 2003). The main component of P. ginseng is the triterpenoid family of saponins (ginsenosides), one of the most commonly studied of which is ginsenoside Rb1 that has been shown to act as a central nervous system inhibitory agent and enhance immune function. Additionally, the ginsenoside Rc has been found to promote serum protein synthesis and plasmin activation, and Rf to inhibit pain (Yoon et al., 1997). P. ginseng also contain phenolic compounds, essential oils, and alkaloids, and these, among other polysaccharides of P. ginseng, are known to have anticancer properties and improve immune function (Kim et al., 1990).
Owing to their slow growth rate, wild ginseng plants are not ready for harvest, and therefore commercial production, until they are 10 - 15 years old (Wang et al., 1998). In addition to this, the small size of P. ginseng limits its cultivation yield. Currently, research is being conducted to cultivate wild ginseng in aseptic culture facilities using biotechnology to secure high root production (Son and Hall, 1990; Yoo et al., 2003). Ginseng cultured roots are produced by separating induced callus from the tissue of natural wild ginseng, which are then usually cultured in a bio cultivator for a period of approximately 45 days (Jeong et al., 2005). In order to induce useful secondary metabolites such as alkaloid and triterpenoid, jasmonic acid has been used to increase specific ginsenosides using cell culture (Yu et al., 2002).
Prior research has shown that when a large amount of wild ginseng root muscle is produced using a bioreactor, and the remaining culture is recycled to grow radish, the components present in the culture activate the growth of radish (Kwon et al., 2009). Recently, there has been reported of biological activity by fermentation treatment with roots of wild ginseng cultured through a bioreactor (Kim et al., 2016a). Other research has found that application of wild ginseng culture broth using proteomics technology can produce high quality, and disease resistant, pigs (Seol et al., 2011). In addition, discarded wild ginseng cultures have been used to supplement the diet of laying hens (Park et al., 2005). However, to date there has been limited research on the application of discarded ginseng culture fluids in animal rearing, and only a few reported cases of application in plant cultivation. Despite the presence of many active ingredients in discarded ginseng tissue culture broth, there is limited research on its industrial application.
Therefore, this study was conducted to identify whether the discarded P. ginseng culture broth could be effectively used in promoting the growth of leafy vegetables.
METHODS AND MATERIALS
1. Analysis of wild P. ginseng tissue culture components and growth conditions of leafy vegetables
Wild P. ginseng (C. A. Meyer) culture broth samples were concentrated under reduced pressure to conduct HPLC analysis. MeOH was used as the final injection solvent. 10 ㎕ of the culture broth were then taken and analyzed using HPLC. The HPLC instrument used was a Shimadzu LC-10AT system (Shimadzu, Kyoto, Japan), and the column used was 4.6 ㎜ × 150 ㎜ reverse phase Eclipse XDB-C18 3.5 ㎛. Flow rate was set at 1.0 ㎖/min, and the UV absorbance detector was fixed at 203 ㎚ for the experiment.
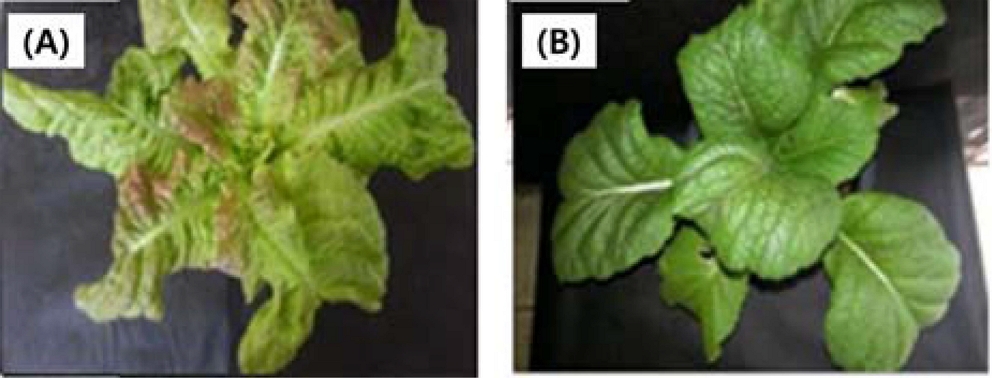
Sixteen standard ginsenosides were employed in the study; Rg1, Re, Rf, Rg2, Rh1, Rb1, Rc, Ro, Rb2, Rd, Rk3, Rh4, Rh2, Rg3, Rk1, and Rg5 (Sigma-Aldrich Co., St. Louis, MO, USA) Seeds of leafy vegetables, fresh red-cotton lettuce (Lactuca sativa) and Asian red mustard (Brassica juncea), were planted in pots and were then treated with the diluted culture solution (dilutions were 1 : 20, 1 : 50, and 1 : 100) (Fig. 1). Treatments were applied twice weekly for a period of eight weeks.
2. Total phenolic content of leafy vegetables treated with P. ginseng culture broth
The color change reagent, which reacts specifically with phenolic substances, was measured using Folin-Ciocalteau reagents (Sigma-Aldrich Co., St. Louis, MO, USA) (Taga et al., 1984). Seven microliters of the Folin-Ciocalteau reagent was added to 14 ㎕ of the sample and stabilized for a 5 min period. Absorbance was then measured at 725 ㎚ (Multiskan FC Microplate Photometer, Thermo Fisher Scientific Inc., Waltham, MA, USA).
Gallic acid was prepared as a standard, and the photo of Fig. 2 was treated by P. ginseng culturing broth for the two leafy vegetables tested, L. sativa and B. juncea.
3. Total flavonoid content of leafy vegetables treated with P. ginseng culturing broth
Twenty microliters of 10% aluminum nitrate (w/v) and 20 ㎕ of 1 M potassium acetate (w/v) were added to 100 ㎕ of the sample. 86 ㎖ of 80% EtOH was then added and stabilized for 40 min at 25℃ (Kim et al., 2016a).
Absorbance was measured at 415 ㎚ using a UV-VIS spectrophotometer (Multiskan FC Microplate Photometer, Thermo Fisher Scientific Inc., Waltham, MA, USA) against the standard material, quercetin. Results obtained are shown in Fig. 3.
4. Antioxidant activity of leafy vegetables treated with P. ginseng culture broth, as estimated by DPPH free radical scavenging
Antioxidant activity was measured by modifying DPPH (1,1-diphenyl-2-picryl-hydrazyl) using the free radical scavenging method (Blois, 1958). Samples concentrated in each treatment group were prepared by diluting samples using 80% MeOH extracted solvent to concentrations of 10, 50, 100, 200, and 500 ppm, respectively. 100 ㎕ of the sample in 96-well plate was added to treatments along with 100 ㎕ of 0.15 mM DPPH solution.
Samples were then left to react for 30 min at room temperature in the dark. After the reaction was complete the absorbance of samples was measured at 517 ㎚ using a UV spectrophotometer. Following absorbance measurement, the concentration of RC50 (㎍/㎖), which reduces the value of the control group without the compound by 50%, was identified.
5. Antioxidant activity of leafy vegetables treated with P. ginseng culture broth, as estimated by ABTS free radical scavenging
Equal amounts of 7.4 mM ABTS (Sigma-Aldrich Co., St. Louis, MO, USA) and 2.6 mM potassium persulfate (Daejung Chemicals and Metals Co., Ltd., Siheung, Korea) were left to react for 24 h at room temperature in the dark to form radicals. The solution was then diluted using phosphate-buffered saline (pH 7.4) to obtain an absorbance of 0.73 ± 0.03 units at 732 ㎚ using a spectrophotometer. Following dilution, 50 ㎕ of the extract was mixed with 950 ㎕ of ABTS and left to react for a further 10 min at room temperature in the dark.
Absorbance was subsequently measured at 732 ㎚ (Re et al., 1999). After absorbance measurement, RC50 (㎍/㎖) concentration was identified and compared with ascorbic acid concentration (a water-soluble vitamin used as an antioxidant).
6. Antimicrobial activity of leafy vegetables treated with P. ginseng culture broth
To investigate antimicrobial activity the serial two fold dilution method was employed (Kobayashi et al., 1993). Gram-positive Bacillus cereus, and Gram-negative Eschericia coli and Salmonella enteritidis (Korean collection for type cultures, South Korea), bacteria were used for the experiment. 10 ㎖ of the cell suspension of each test specimen was inoculated in a conical tube and the sample cultured using shaking incubator at 120 rpm for a period of 12 h or more at each growth temperature. The culture solution was diluted 100 fold in each growth medium in preparation of antimicrobial testing.
The sample was then placed in the first well of a 96-well micro assay plate and the prepared cell suspension was dispensed up to eight times (this was done by dividing the diluted cell suspension twice). The solution was subsequently cultured for 24 h at 37℃ in an incubator, and the extent of bacterial growth was visually observed. Benzoic acid was used as a positive control and distilled water was used as a negative control. Minimum inhibitory concentration (MIC) was measured to inhibit the growth of bacteria.
7. General ingredient analysis of leafy vegetables treated with P. ginseng culture broth
The general constituents of the leafy vegetables identified by the dilution treatments and their bioactivity were analyzed using the AOAC method (AOAC, 1990).
Moisture content is measured, atmospheric pressure drying method at 105℃ and crude protein content is used micro-Kjeldahl method (Kjeltec protein analyzer, Foss Tecator AB, Hillerød, Denmark). Crude fat content is used soxhlet extraction method into the sample. Samples were analyzed using ethanol extraction. The ash content was directly incinerated, and the sample was preliminarily carbonized in an electric furnace and then incubated at 550℃ - 600℃ for a minimum of 12 h making the sample grayish in color. After cooling, the sample content was calculated and represented as a percentage of the sample weight. The carbohydrate content was measured by subtracting water.
8. Inorganic element analysis of leafy vegetables treated with P. ginseng culture broth
The reaction was stopped by treating the 0.5 g powder sample with 7 ㎖ HNO3 over a 6 h period. The sample was then treated with H2O2. The solvent was completely removed from the sample by raising the temperature at 80℃, 130℃, 150 ℃ and 180℃ increments for 5 min using a microwave (Samsung, Suwon, Korea).
Following removal of the solvent ICP-OES (OPTIMA7300DV, Perkinelmer Inc., Waltham, MA, USA) spectroscopy was performed by adding tertiary distilled water to the sample (Osborne and Voogt, 1981). A calibration curve was then prepared by diluting the standards (As, Cu, Ca, Fe, Mn, Mg, Zn, V, Se) for in organic element analysis, and then calculated by substituting the area ratio at the wavelength of the inorganic element into the calibration curve.
RESULTS AND DISCUSSION
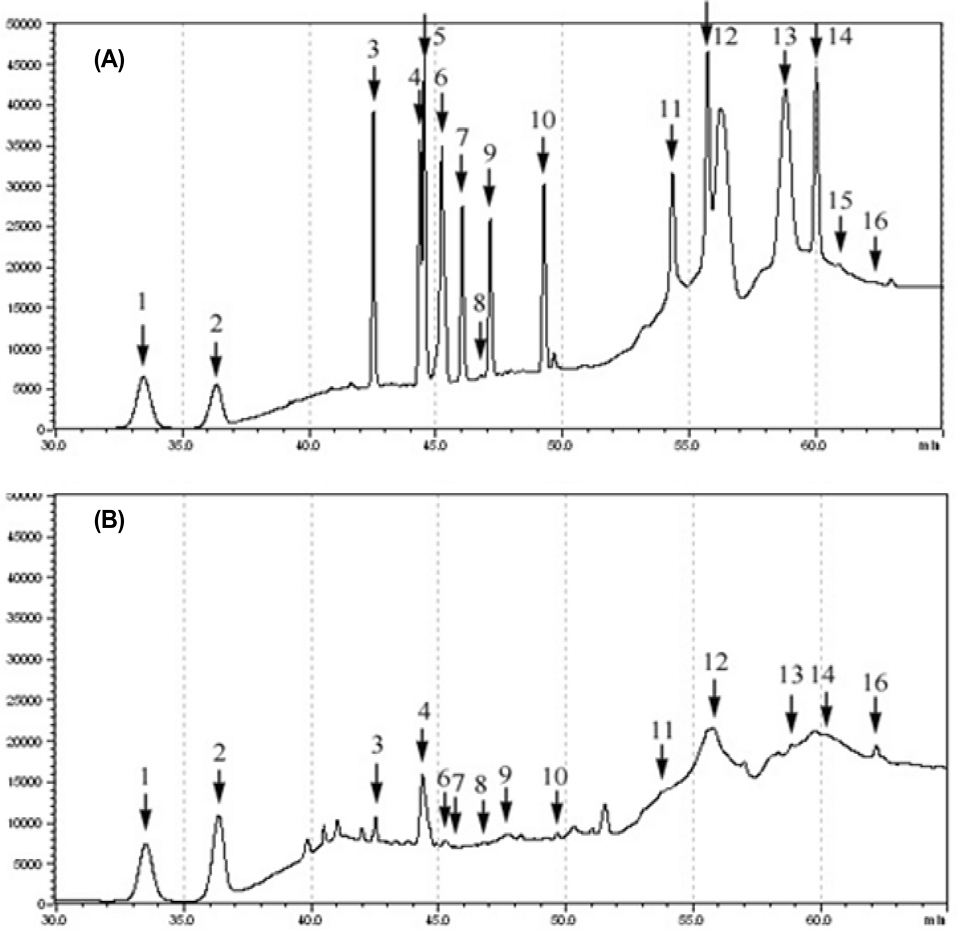
1. HPLC analysis of ginsenoside in wild ginseng culture broth cultured in a bioreactor
The ginsenosides in wild ginseng (Panax ginseng C. A. Meyer) cultures were identified using HPLC (Fig. 2). All ginsenosides except for Rh1 and Rk1 were detected but with varying concentrations. Ginsenoside Rg5 had the highest content of all ginsenosides measured at 16.937 ㎍/g (Table 1).
Prior research has found wild ginseng cultures contain about 2% wild ginseng and more than 10% saponins (Bae et al., 2003). Additionally, many studies have investigated the efficacy of saponins, which make up the most abundant component of wild ginseng cultures (Kim et al., 2002; Santos et al., 2002). As research suggests cultures containing ginsenosides contain effective ingredients that can improve plant growth and functionality, it may be that products can be developed using these cultures for agricultural application.
2. Analysis of total phenolic and flavonoid content of leafy vegetables treated with wild ginseng culture broth
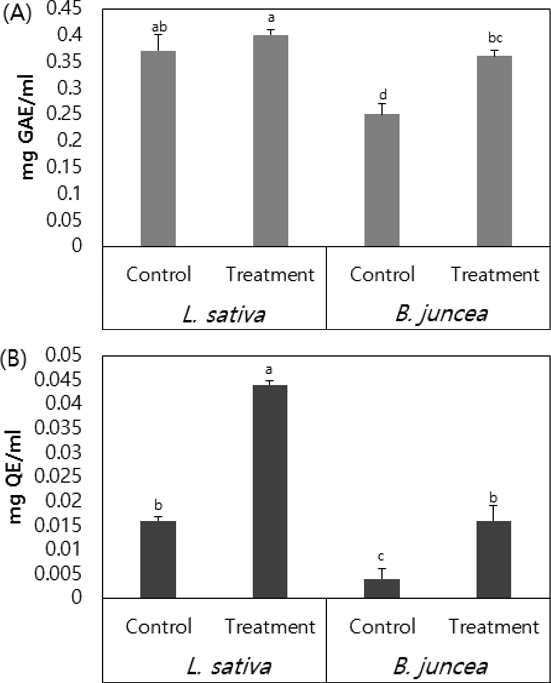
Phenolic substances are widely distributed as secondary metabolites in plants and are known to be involved in various biological activities of phenolic hydroxyl groups. In addition, it has involved in a bioactive function such as antimicrobial effect by inducing growth inhibition by interacting with microbial cells (Moon et al., 2004). In the current study it was found that total phenolic content was 0.40 ㎎·GAE/㎖ for treated L. sativa, which was higher than that in the control (0.37 ㎎·GAE/㎖). The B. juncea treatment group measured a phenolic content of 0.36 ㎎·GAE/㎖, which was significantly higher than that of the control (0.25 ㎎·GAE/㎖) (Fig. 3A).
Flavonoids are known to affect biochemical activities such as inhibition of lipid peroxide production (Middleton and Kandaswami, 1994). A study investigating flavonoid content in wild ginseng found wild ginseng cultured root extracts fermented with microorganisms had a total flavonoid content of 100 ㎍·QE/㎖ or more (Kim et al., 2016b). In the current study Flavonoid content was 0.044 ㎍·QE/㎖ in treated L. sativa plants, which was 2.6 fold higher than that of the control. Treated B. juncea had a flavonoid content of 0.016 ㎎·QE/㎖, 3.8 fold higher than that of the control (0.004 ㎎·QE/㎖) (Fig. 3B). Although flavonoid content found in culture broth is less than the amount of flavonoids found in wild ginseng culture root, its detection is important given the availability of wild ginseng culture broth. As a result of analyzing the wild ginseng culture broth, since the ginsenoside content is present, it is considered that the phenol and flavonoid contents of the leafy vegetables are increased when applied to leafy vegetables.
3. Free radical scavenging activity of leafy vegetables treated with wild ginseng culture
DPPH is a representative reactant used to measure antioxidant activity; it is a purple compound that exhibits specific light absorption at 517 ㎚. It is considered a very stable free radical and is used as a substrate for measuring activity of antioxidants (Jeon et al., 2009).
In a study by Jang et al. (2008) that compared DPPH radical scavenging activity of wild ginseng, wild-cultivated ginseng, and ginseng extracts, wild ginseng was found to have the lowest antioxidant activity. It has also been found that when the concentration of wild ginseng culture root extract is increased, electron donating ability also proportionally increases (Kim et al., 2016b).
As a result of free radical scavenging activity of the leafy vegetables treated with wild ginseng culture broth used to produce ginseng culture roots in the present study, the antioxidant activity was increased in treated L. sativa by reducing the RC50 value to 255.53 ± 0.15 ㎍/㎖ in the L. sativa treatment when compared with 311.12 ± 0.13 ㎍/㎖ in the control. The B. juncea treatment group showed an increased antioxidant activity by reducing the RC50 value 1.5 times to 859.33 ± 0.10 ㎍/㎖ when compared with the control measured at 1274.75 ± 0.08 ㎍/㎖ (Table 2). This result is thought to be due to the increase in antioxidant functional substance content in the leafy vegetables as a result of treatment with the culture broth.
Free radical scavenging activity by DPPH1) method of leafy vegetables treated with P. ginseng C. A. Meyer tissue culture broth.
As a result of the ABTS free radical scavenging method, the antioxidant activity of L. sativa was increased to 750.00 ± 0.10 ㎍/㎖, which was notably lower than that of the control at 900.60 ± 0.04 ㎍/㎖. Antioxidant activity was also increased in treated B. juncea, which measured 825.40 ± 0.05 ㎍/㎖ an RC50 value 1.8 times lower than that in the control (1521.11 ± 0.03 ㎍/㎖) (Table 3). In the water extract of ginseng, there is no electron donating activity, and in the fermented ginseng extract, there is an electron donating activity, and thus it shows differences according to materials and extraction solvent (Kim et al., 2007; Doh et al., 2010).

Free radical scavenging activity by ABTS1) method of leafy vegetables treated with P. ginseng C. A. Meyer tissue culture broth.
Given the free radical scavenging activity was found to be higher than that of the control group for both leafy vegetables treated for the present study, wild ginseng culture broth may have application in growing leaf vegetables.
4. Antimicrobial activity of leafy vegetables treated with wild ginseng culture broth
There have been some reports of antimicrobial activity assays of wild ginseng; however, few studies have reported antimicrobial activity assays using wild ginseng culture broth (Kim et al., 2016a).
In the current study, the results of antimicrobial analysis using the two fold dilution method showed treated L. sativa had an increase of 1,250 ㎍/㎖ growth inhibitory activity against Salmonella enteritidis, a gram-negative bacterium. Inhibition of Bacillus cereus was also identified to 625 ㎍/㎖ in B. juncea (Table 4). It may be that antimicrobial activity, identified in the treatment of the culture broth, could have various potential applications.
5. Analysis of general components of leafy vegetables treated with wild ginseng culture
Prior research has identified to contain 9.08% water, 61.72% carbohydrates, 17.36% crude protein, 0.23% crude lipid, and 10.90% crude ash in a 100 g (wet weight basis) sample in wild ginseng cultured roots (Park et al., 2012).
In the current study, the moisture content of L. sativa treated with wild ginseng culture broth was found to be lower than that of the control group (93.8%). Crude ash, crude protein, crude lipid, carbohydrate, and calorie content were 1.2%, 2.3%, 0.3%, and 2.4%, respectively. The moisture content of B. juncea was also found to be less than that of the control, at 91.0%. Conversely; ash, crude protein and sulcus content were 0.2% (crude ash) and 0.6% (crude protein) higher than those of the control for treated B. juncea. However, crude lipid and carbohydrate content were the same as that of the control, at 0.2% and 4%, respectively (Table 5).
These findings indicate that the ash and crude protein content of the leafy vegetables treated with culture broth were higher than those of the control suggesting the protein source may be enhanced by the increase of crude protein content when the treated leafy vegetables are ingested.
6. Inorganic element analysis of leafy vegetables treated with wild ginseng culture
The results of the current study found there was a change in inorganic elements of leafy vegetables treated with wild ginseng culture. L. sativa showed an increase in Ca, Mn, Mg, V, and Se content; notably, Ca increased 1.5 fold and Mg increased 1.3 fold. Conversely, the Zn content was decreased in treated L. sativa, as was harmful As, which was decreased by 0.106 ㎎/ℓrelative to the control. In the B. juncea treatment group Cu, Ca, Fe, Mn, Mg, Zn, V, Se content all increased; Fe increased 3 fold, Mn 1.8 fold, and Se 2 fold. Harmful As also decreased to 0.132 ㎎/ℓ relative to the control (Table 6).
Results also indicated inorganic element content of wild ginseng root was the highest in P, Ca, and Mg consecutively (Park et al., 2012). Inorganic elemental analysis of K and P was not performed in the current study, but calcium and magnesium were found to be higher than other elements analyzed, somewhat in line with a study by Park et al. (2012).
The difference in the transition content and the transfer rate of inorganic elements, that are transferred according to the treatment of wild ginseng culture broth, is postulated to be suppressed or accompanied by the presence or transitions of other specific inorganic elements. It is thought this has various effects, such as on crop growth and body absorption, by increasing the content of rare elements (e.g., V and Se), by increasing vitamin C resistance, and through antagonism and antioxidant activity with heavy metals.
From this study, the field of application of wild ginseng cultivation broth can be used in various industries that are the basis of new bio-materials by increasing functionality due to the growth promotion and the change of physiological activity in agriculture.
Acknowledgments
This work was carried out with support of the Bioherb Research Institute, Kangwon National University, Republic of Korea.
References
- Association of Official Analytical Chemists(AOAC). (1990). Official Methods of Analysis, (15th ed.). Association of Official Analytical Chemists, Washington DC, USA. p.788.
-
Bae GS, Nam HP, Kim HS, Lee SG, Choi HS, Min WK, Joo JW, Maeng WJ and Chang MB. (2003). Effects of the artificial culture medium of wild ginsengs on rumen fermentation characteristics in vitro. Journal of Animal Science and Technology. 45:987-996.
[https://doi.org/10.5187/JAST.2003.45.6.987]

-
Blois MS. (1958). Antioxidant determinations by the use of a stable free radical. Nature. 181:1199-1200.
[https://doi.org/10.1038/1811199a0]

- Doh ES, Chang JP, Lee KH and Seong NS. (2010). Ginsenoside change and antioxidation activity of fermented ginseng. Korean Journal of Medicinal Crop Science. 18:225-265.
-
Jang HY, Park HS, Kwon KR and Rhim TJ. (2008). A study on the comparison of antioxidant effects among wild ginseng, cultivated wild ginseng, and cultivated ginseng extracts. Journal of Pharmacopuncture. 11:67-78.
[https://doi.org/10.3831/KPI.2008.11.3.067]

-
Jeon GI, Yoon MY, Park HR, Lee SC and Park EJ. (2009). Neuroprotective activity of Viola mandshurica extracts on hydrogen peroxide-induced DNA damage and cell death in PC12 cells. Annals of the New York Academy of Sciences. 1171:576-582.
[https://doi.org/10.1111/j.1749-6632.2009.04889.x]

- Jeong HS, Kang TS, Woo KS, Paek KY, Yu KW and Yang SJ. (2005). Effects of cultured wild ginseng roots on the alcoholic fermentation. Korean Journal of Food Preservation. 12:402-410.
-
Kim CJ, Seong ES, Yoo JH, Lee JG, Kim NJ, Choi SK, Lim JD and Yu CY. (2016a). Biological activity of Panax ginseng C. A. Meyer culture roots fermented with microorganisms. Korean Journal of Medicinal Crop Science. 24:191-197.
[https://doi.org/10.7783/KJMCS.2016.24.3.191]

- Kim CS, Park JB, Kim KJ, Chang SJ, Ryoo SW and Jeon BH. (2002). Effect of Korea red ginseng on cerebral blood flow and superoxide production. Acta Pharmacologica Sinica. 23:1152-1156.
-
Kim KT, Yoo KM, Lee JW, Eom SH, Hwang IK and Lee CY. (2007). Protective effect of steamed American ginseng(Panax quinquefolius L.) in V79-4 cells induced by oxidative stress. Journal of Ethnopharmacology. 111:443-450.
[https://doi.org/10.1016/j.jep.2007.01.004]

-
Kim SH, Lee SY, Cho SM, Hong CY, Park MJ and Choi IG. (2016b). Evaluation on anti-fungal activity and synergy effects of essential oil and their constituents from Abies holophylla. Journal of Korean Wood Science and Technology. 44:113-123.
[https://doi.org/10.5658/WOOD.2016.44.1.113]

-
Kim YS, Kang KS and Kim SI. (1990). Study on antitumor and immunomodulating activities of polysaccharide fractions from Panax ginseng: Comparison of effects of neutral and acidic polysaccharide fraction. Archives Pharmacal Research. 13:330-337.
[https://doi.org/10.1007/BF02858168]

-
Kobayashi A, Koguchi Y, Takahashi S, Kanzaki H and Kawazu K. (1993). ST-1, a novel radical scavenger from fungus F-124. Bioscience, Biotechnology, and Biochemistry. 57:1034-1046.
[https://doi.org/10.1271/bbb.57.1034]

- Kwon SS, Seong ES, Gho EJ, Ahn HS, Kim BJ, Lee JY, Lee JK and Yu CY. (2009). Effect of recycled liquid medium on the growth of Raphanus sativus L. after wild Panax ginseng culture by bioreactor. Journal of Agriculture and Life Sciences. 20:55-60.
- Middleton EJ and Kandaswami C. (1994). Potential health-promoting properties of citrus flavonoids. Food Technology. 48:115-119.
- Moon JS, Kim SJ, Park YM, Hwang IS, Kim EH, Park JW, Park IB, Kim SW, Kang SG, Park YK and Jung ST. (2004). Antimicrobial effect of methanol extracts from some medicinal herbs and the content of phenolic compounds. Korean Journal Food Preservation. 11:207-213.
- Osborne DT and Voogt P. (1981). The analysis of nutrients in foods. In Stwart GF. et al.(eds.). Food Science and Technology. Academic Press. London, England. p.169.
- Park JH, Shin OS and Ryu KS. (2005). Effect of feeding wild ginseng culture by-products on performance and egg quality of laying hens. Korean Journal of Poultry Science. 32:269-273.
-
Park SJ, Yoo SM and Kim YE. (2012). Nutritional characteristics and screening of biological activity of cultured wild ginseng roots. Korean Journal of Food and Nutrition. 25:729-736.
[https://doi.org/10.9799/ksfan.2012.25.4.729]

-
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M and Rice-Evans C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine. 26:1231-1237.
[https://doi.org/10.1016/S0891-5849(98)00315-3]

-
Santos WR, de Lima VMF, de Souza EP, Bernardo RR, Palatnik M and de Sousa CBP. (2002). Saponins, IL12 and BCG adjuvant in the FML-vaccine formulation against murine visceral leishmaniasis. Vaccine. 21:30-43.
[https://doi.org/10.1016/S0264-410X(02)00444-9]

- Seol JW, Chae JS, Kang HS, Kang CS, Ihn DC and Park SY. (2011). Proteome analysis of pigs fed with tissue culture medium waste after harvest of Korean wild ginseng. Journal of Veterinary Clinics. 28:75-80.
-
Son SH and Hall RB. (1990). Multiple shoot regeneration from root organ cultures of Populus grandidentata×P. grandidentata. Plant Cell Tissue and Organ Culture. 20:53-57.
[https://doi.org/10.1007/BF00034757]

-
Taga MS, Miller EE and Pratt DE. (1984). Chia seeds as a source of natural lipid antioxidants. Journal of the American Oil Chemists’ Society. 61:928-931.
[https://doi.org/10.1007/BF02542169]

- Wang ZH, Li CH, Zhang CG and Wang XD. (1998). Study on the wild ginseng and cultured ginseng in northeast of China. Proceedings of the Ginseng Society Conference. p.109-114.
- Yoo BS, Chang MS and Byun SY. (2003). Characterization of cell cultures and ginsenoside production by cultured ginseng and wild mountain ginseng. Korean Journal of Biotechnology and Bioengineering. 18:133-139.
- Yoon SR, Nah JJ, Shin YH, Nam KY, Kim SK and Nah SY. (1997). Ginsenoside Rf induces differential antinociception: From cell to antinociception. Proceedings of ’97 Korea-Japan Ginseng Symposium. p.166-180.
-
Yu KW, Gao W, Hahn EJ and Paek KY. (2002). Jasmonic acid improves ginsenoside accumulation in adventitious root culture of Panax ginseng C. A. Meyer. Biochemical Engineering Journal. 11:211-215.
[https://doi.org/10.1016/S1369-703X(02)00029-3]