
Osteoprotective Effect of Extract from Cirsium japonicum var. ussuriense in Ovariectomized Rats
© The Korean Society of Medicinal Crop Science All rights reserved
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License ( http://creativecommons.org/licenses/by-nc/3.0 ) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
This study was carried out to investigate the effects of the Cirsium japonicum var. ussuriense (C. japonicum) extract on serum level of hormones from induced osteoporosis by ovariectomized rats. Two month-old rats were ovariectomized (OVX), remained untreated for 8 weeks, and were subsequently administered C. japonicum (200 ㎎/㎏) every day for 8 weeks. We examined the effects of treated C. japonicum on ovariectomy-related changes in Insulin-like Growth Factors (IGF), Insulin-like Growth Factor binding protein-3 (IGBF-3), Estrogen, Calcium, and Phosporus. After 8 weeks, the serum levels of IGF-I, -II, and IGFBP-3 were higher presented as compared to the other two groups (P < 0.05), in the C. japonicum extract treatment on OVX rats. There were differences between OVX and C. japonicum extract treated OVX rats in serum levels of Ca2+, but Ca2+ levels for the normal group was higher than for the other two groups. The C. japonicum extract increased both serum IGFs and IGFBP-3 levels on induced osteoporotic rat by ovariectomized. Thus, these results revealed that the C. japonicum extract is a possible role for improvement of osteoporosis induced-ovariectomized rats and has a great potential as an alternative tool for the treatment of osteoporosis.
Keywords:
Cirsium japonicum, Osteoporosis, Ovariectomy, SerumINTRODUCTION
Osteoporosis is a disease characterized by low bone mass and deterioration of bone tissue. This leads to increased bone fragility and risk of fracture. Osteoporosis is often known as “the silent thief” because bone loss occurs without symptoms (Christiansen, 1993). A decrease in in women at the time of menopause and a decrease in testosterone in men is another major cause of bone loss. After menopause, the normal balance between bone formation and resorption is disrupted : osteoclasts (giant multi-nucleated cells) become more active, decreasing bone mass and increasing the chance of fracture. It is now known that postmenopausal osteoporosis should be regarded as a product of inflammatory disease triggered by estrogen deficiency (Chung et al., 2006). Ovariectomy (OVX) in the rat results in bone loss and microarchitecture deterioration (Ammann et al., 1996) and is the most commonly used animal model of post-menopausal osteoporosis (Turner et al., 2001). Although hormone replacement therapy has been proven to be efficacious in preventing bone loss, yet, it is not desirable to many women due to its side effects (Epstein, 2006). Estrogen deficiency results in a marked increase in proinflammatory cytokines, interleukin-1 (IL-1), interleukin-6 (IL-6), tumor necrosis factor-a (TNF-a), and macrophage colony- stimulating factor (McLean, 2009). TNF-a, IL-1b, and IL-6 stimulate osteoclast differentiation independently or in synergistic fashion (Jiro et al., 2011). Because estrogen deficiency has been recognized as the key factor of osteoporosis, hormone replacement therapy (HRT) was used as an effective strategy for prevention and treatment of osteoporosis in postmenopausal women.
Recently, therapy for osteoporosis is aimed at the prevention of further bone loss, primarily by inhibiting bone resorption. Particularly, insulin-like growth factors (IGF) and their binding proteins (IGFBP), which could modulate IGF's actions, might play essential roles in bone formation (Nilsson et al., 1994; Rosen et al., 1994). In serum, most IGF-I (70-80%) forms a 150-kD complex by binding with IGFBP-3. The serum IGFBP-III level is considered to positively regulated by GH and/or IGF-I (Rosen and Pollak, 1999; Stewart and Rotwein, 1996). IGFBP-III acts as a regulator of growth-dependent IGF-I signaling through the enhancement of IGF-I stability in serum (Binoux and Hossenlopp, 1988). A major part of bound IGF-I is connected to IGFBP-III (Baxter, 1993). Low estrogen concentrations are known to increase the secretion of subsequent IGF-I synthesis during early puberty. Estrogens initiate the pubertal growth spurt and stimulate skeletal growth. Hence, sex steroid-related changes in GH and IGF-I secretion may impact on bone size and cross-sectional area (Juul, 2001). The popularity of herbal medicines is attributable to their easy take, therapeutic efficacy, relatively low cost, and assumed absence of toxic side effects (Park et al., 2013). The use of natural products as an alternative treatment has been on the rise in the last few decades C. japonicum, a wild perennial herb which belongs to Asteraceae family, has long been used as a natural medicine for treatment of hypertension, traumatic hemorrhage, and inflammation (Kim et al., 2010). Recent studies have found that the water extracts of C. japonicum induce the activation of estrogen receptors and has estrogenic effects (Park et al., 2008) and has been prescribed in the treatment of liver cancer (Lee et al., 2003), uterine cancer, and leukemia (Yin et al., 2008). But since now no studies have been carried out to evaluate whether C. japonicum has an antiosteoporosis activity in rats. The present research was conducted to investigate the effect of C. japonicum on postmenopausal osteoporosis in ovariectomized rats.
MATERIALS AND METHODS
1. Experimental animals
The Sprague-Dawley rats were purchased from the Jungang Lab animal co, South Korea. A total of 60 female rats, 2-month-old, weighing 180-200g were randomly assigned into 6 groups (10 animals/group). (i) sham, (ii) OVX_control, (iii) 17β-estradiol, 17β-estradiol (E2) 30 mcg/kg/24 hour, (Sigma, St. Lois, MO, USA) C. japonicum. Except 17β-estradiol (positive control), which was administered every three days for 8weeks, each treatment sample was administered once daily p.o. at a dose of (iv) 100, (v) 200 and (vi) 400mg/kg for 8weeks. The animals were housed in stainless steel cages having wire mesh bottom (3 animals per cage) in a lightcontrolled (12 : 12 hours light-dark cycle) room with a constant temperature (25°C). All the rats had free access to food and water. The animals in group I were normal group. To induce menopause in rats, ovariectomies were performed in group I and group II. Ovariectomies were done under ketamine HCl (100mg/kg) and xylazine (3mg/kg) anesthesia. During 8 weeks, the animals of group I and II received solvent vehicle daily, whereas those of group III were administered C. japonicum orally (100, 200 and 400mg/kg) daily for eight weeks. Body weight was determined weekly.
2. Preparation of C. japonicum extraction
The aerial parts of C. japonicum were collected on July 20, 2013 from Imsil herbal farming the Act at Imsil in Chonnam, Republic of Korea, and then specimens were taxonomically identified by an oriental doctor, S. W. Lee at National Institute of Horiticultural & Herbal Science, RDA. We used water extraction because most traditional oriental herbal materials are decocted with boiling water. The crushed plant materials (100 g for each) were kept in a breaker at room temperature (25 ± 1). And it extracted under reflux with distilled water three times. After the extraction, the sample residues were dissolved in a known quantity of water and were repeatedly extracted until the extracts became clear. The obtained extract was then filtered through Whatman No. 1 filter paper and the filtrate was concentrated in a rotary evaporator (Buchi Rotavapor R-215, Flawil, Switzerland) under controlled vacuum. The concentrated extract was then dried in a freeze dryer (Labconco, Free Zone 6 Liter, Kansas City, MO, USA), and yields were 16% (wt/wt) for C. japonicum in the dried state. They kept at 4°C in air tight container until further analysis.
3. Measurement of Femur/body weight
Body and femur weight was measured after C. japonicum seed treatment in osteoporosis induced-ovariectomized rats using chemical balance (Ohaus, Parsippany, NJ, USA).
4. Collection of blood and tissue samples
Serum was isolated from the blood samples by centrifugation at 3000 × g and 4°C for 5 min and stored at −70°C for biochemical measurements. Body and isolated femur weight was measured in osteoporosis inducedovariectomized rats using chemical balance (Ohaus, Parsippany, NJ, USA).
5. Measurement of serum IGF-I, IGF-II and IGFBP-III levels
For the quantitative analysis of the serum IGF-I, IGF-II and IGFBP-III levels, free IGF-I and IGFBP-III enzymelinked immunosorbent assay (ELISA) kits were used according to the manufacturer’s protocols (R&D Systems, Minneapolis, MN, USA).
6. Estrogen, Ca2+ and P assay
IGFBP-III and estrogen were assayed using an immunoradiometric assay kit (Diagnostic Systems Laboratories, Webster, TX, USA). The Ca2+ and P assays used kits (Embiel Co., Gunpo, Korea).
7. Statistical analysis
All the data were presented as mean ± S.E.M. The effects of the different treatments were compared by Student’s t test and were considered significant for values of p < 0.05 and p < 0.01. Post hoc analysis was carried out using Duncan’s Multiple Range Test (DMRT).
RESULTS
1. Animal model
Body weight is shown in Fig.1. Ovariectomy increased the body weight (Normal: 266.60 ± 8.6; ovariectomized: 328.8 ± 7.4; C. japonicum (200mg/kg) treated: 267.5 ± 11.4 (p< 0.001): C. japonicum (400mg/kg) treated: 284.8 ± 16.5). There were no differences in body weight between normal, OVX and C. japonicum-treated until the end of the study, OVX group higher weights than the other groups. Body weight of OVX rats increased about 23.3%, after 8 week surgery. A relatively low dose of C. japonicum, 200mg/kg, more inhibited the increase in body weight than did a high dose, 400mg/kg.
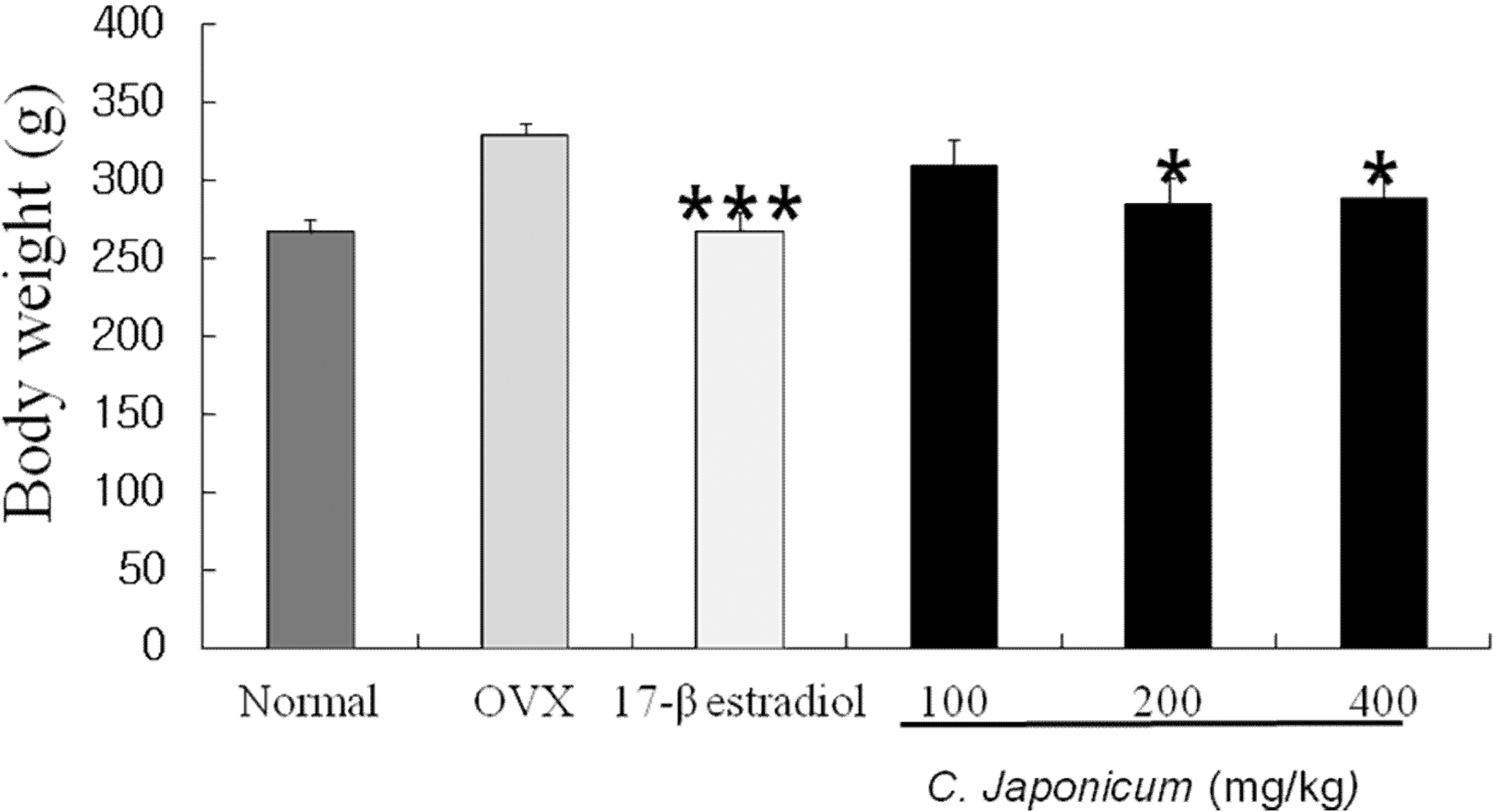
Changes of body weight on OVX and C. japonicum treatment.Values are mean ± S.E.M. The number of animals is 10/group. P-values denote significant difference from the OVX group and 17-β estradiol treated group. ***p < 0.001 vs. the OVX group, *p < 0.05 vs. the OVX group. Normal; Normal and saline treated group, OVX; ovariectomized and saline treated group, C. japonicum; ovariectomized and C. japonicum treated group. 17-β estradiol; ovariectomized and 17-β estradiol (30μg/kg) treated group.
2. Effect of ovariectomy and C. japonicum on serum IGF-I levels
Changes in serum level of IGF-I C. japonicum treatment in osteoporosis induced-OVX rat groups over time are shown in Fig. 2. On 8 weeks after ovariectomy, serum level of IGF-I was significantly higher in the 17-β estradiol and C. japonicum treated OVX group than in the OVX and sham groups (p < 0.01) whereas slight changes were between the normal and OVX groups.
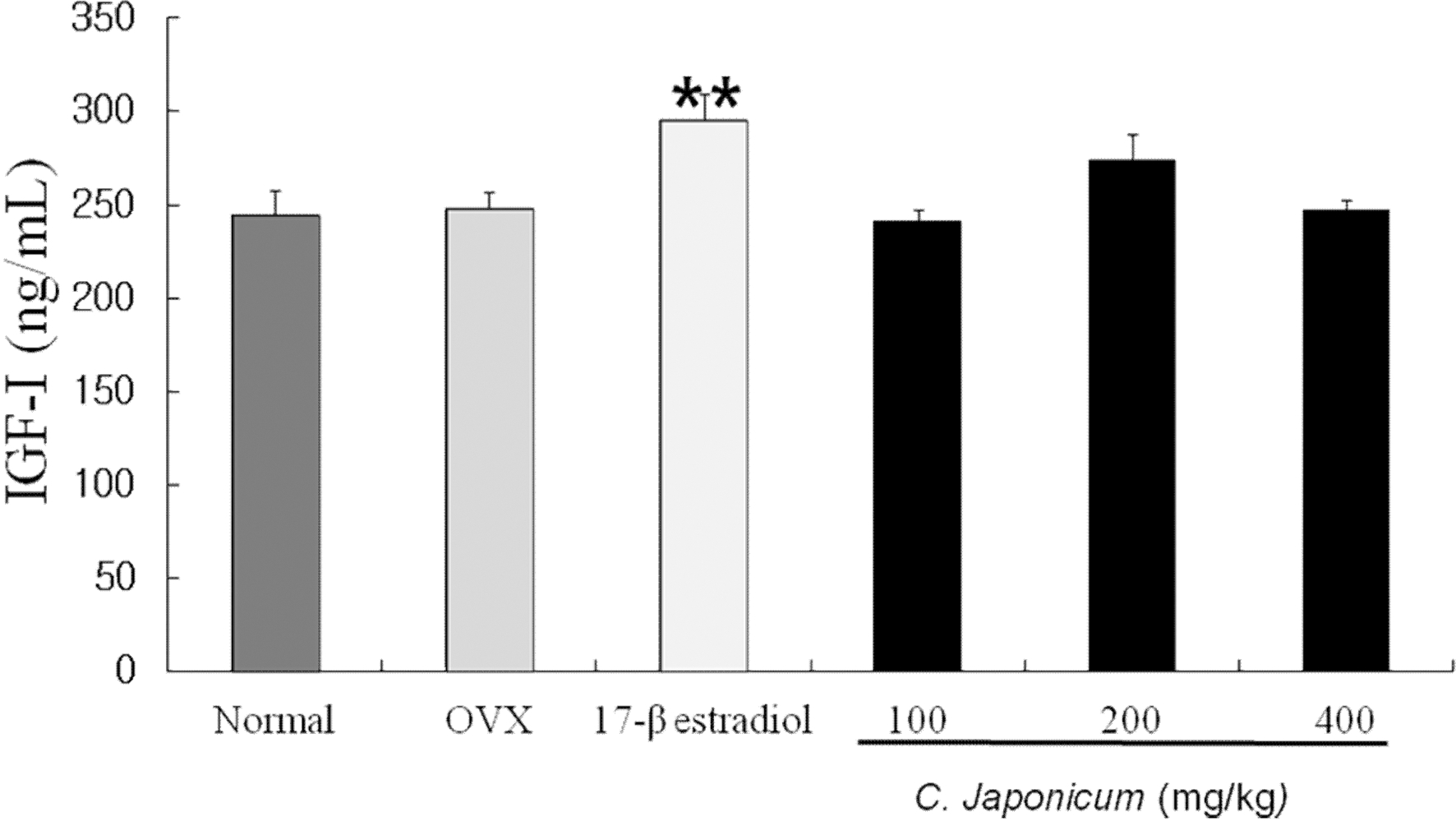
Effects of OVX and C. japonicum on serum IGFI levels determined.Values are mean ± S.E.M. The number of animals is 10/group. P-values denote significant difference from the OVX group and C. japonicum treated group. Normal; Normal and saline treated group, OVX; ovariectomized and saline treated group, C. japonicum; ovariectomized and C. japonicum treated group, 17-β estradiol; ovariectomized and 17-β estradiol (30μg/kg) treated group. **p < 0.01 vs. the OVX group.
3. Effect of ovariectomy and C. japonicum on serum IGF-II levels
Serum level of IGF-II after C. japonicum treatment in osteoporosis induced-OVX rat groups was shown in Fig. 3. On 8 weeks after ovariectomy, serum level of IGF-II on the 17β-estradiol treated group were significantly higher than that of the OVX group (174.4 ng/mL). Serum level of IGF-II after C. japonicum (400mg/kg) treatment was significantly higher than in the OVX groups (p < 0.05) whereas slight changes were between the 100mg/kg and 400mg/kg of C. japonicum treated groups.
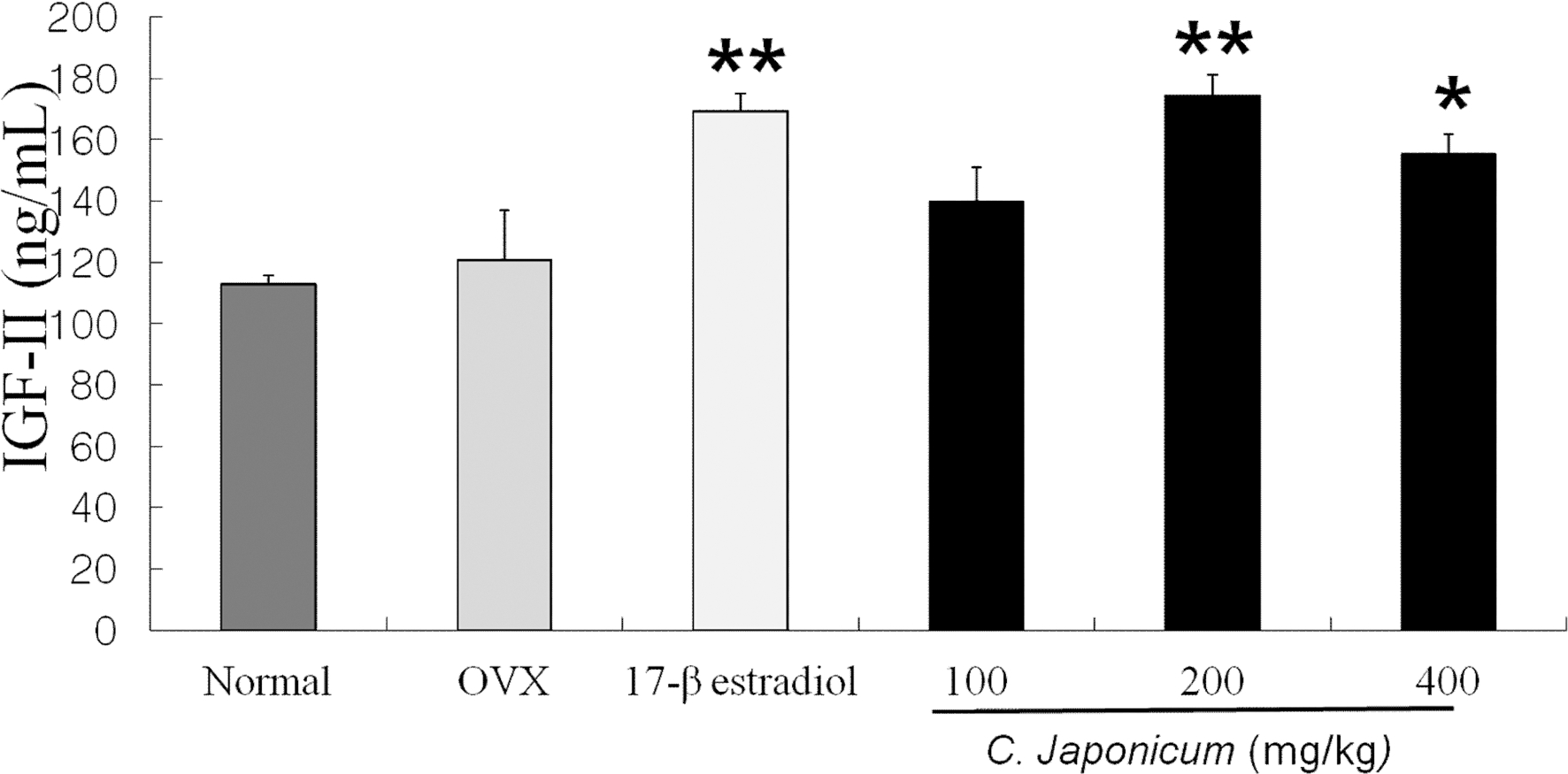
Effects of OVX and C. japonicum on serum IGFII levels determined.Values are mean ± S.E.M. The number of animals is 10/group. P-values denote significant difference from the OVX group and C. japonicum treated group. **p < 0.01 vs. the OVX group, *p < 0.05 vs. the OVX group. Normal; Normal and saline treated group, OVX; ovariectomized and saline treated group, C. japonicum; ovariectomized and C. japonicum treated group. 17-β estradiol; ovariectomized and 17-β estradiol (30μg/kg) treated group. **p < 0.01 vs. the OVX group.
4. Effect of ovariectomy and C. japonicum on serum IGFBP-III and Estrogen levels
IGFBP-III levels after C. japonicum treatment in osteoporosis induced OVX rat groups are shown in Fig 4. On 8 weeks, IGFBP-III levels in the normal group decreased and C. japonicum treatment group significant differences compared with the OVX group (p < 0.05). The serum levels of IGFBP-III in 17β-estradiol (600.1 ± 12.8 ng/ mL) and the 200mg/kg C. japonicum (579.7 ± 8.1 ng/mL) were higher than in OVX group (502.1 ± 30.1 ng/mL), but in C. japonicum (400mg/kg) the serum IGFBP-III decreased to 563.4 ± 9.7 ng/mL. The difference was significant (p < 0.05). Changes in estrogen levels of each group are shown in Fig. 5. On 8 weeks after ovariectomy, serum estrogen levels of each group was difference as a consequence of OVX or the C. japonicum treated group, but there were no differences between C. japonicum treated group and OVX group.
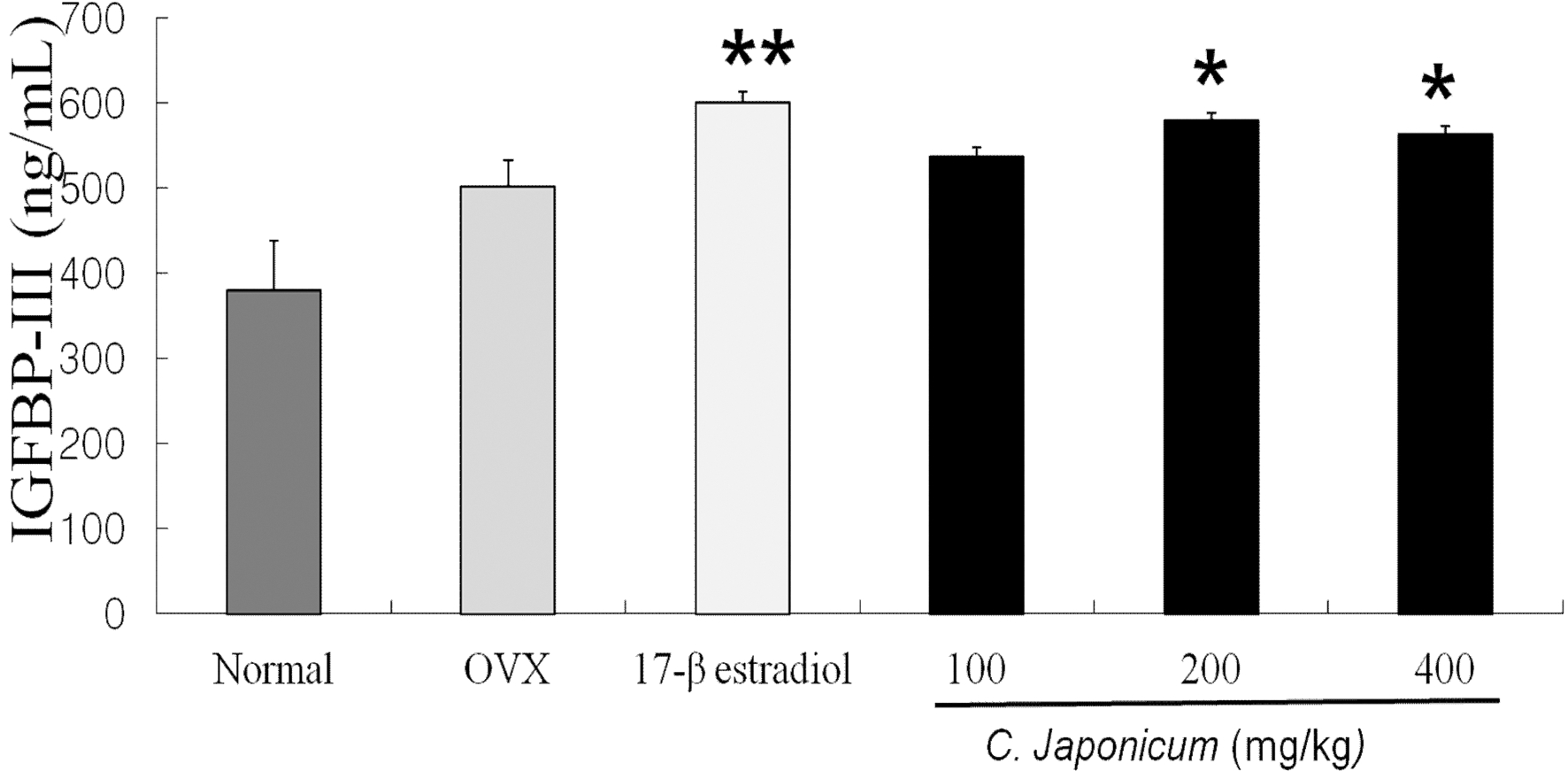
Effects of OVX and C. japonicum on serum IGBPF-III levels determined.Values are mean ± S.E.M. The number of animals is 10/ group. P-values denote significant difference from the OVX group and C. japonicum treated group (p < 0.05). Normal; Normal and saline treated group, OVX; ovariectomized and saline treated group, C. japonicum; ovariectomized and C. japonicum treated group. *p < 0.05 vs. the OVX group. 17-β estradiol; ovariectomized and 17-β estradiol (30μg/kg) treated group. **p < 0.01 vs. the OVX group.
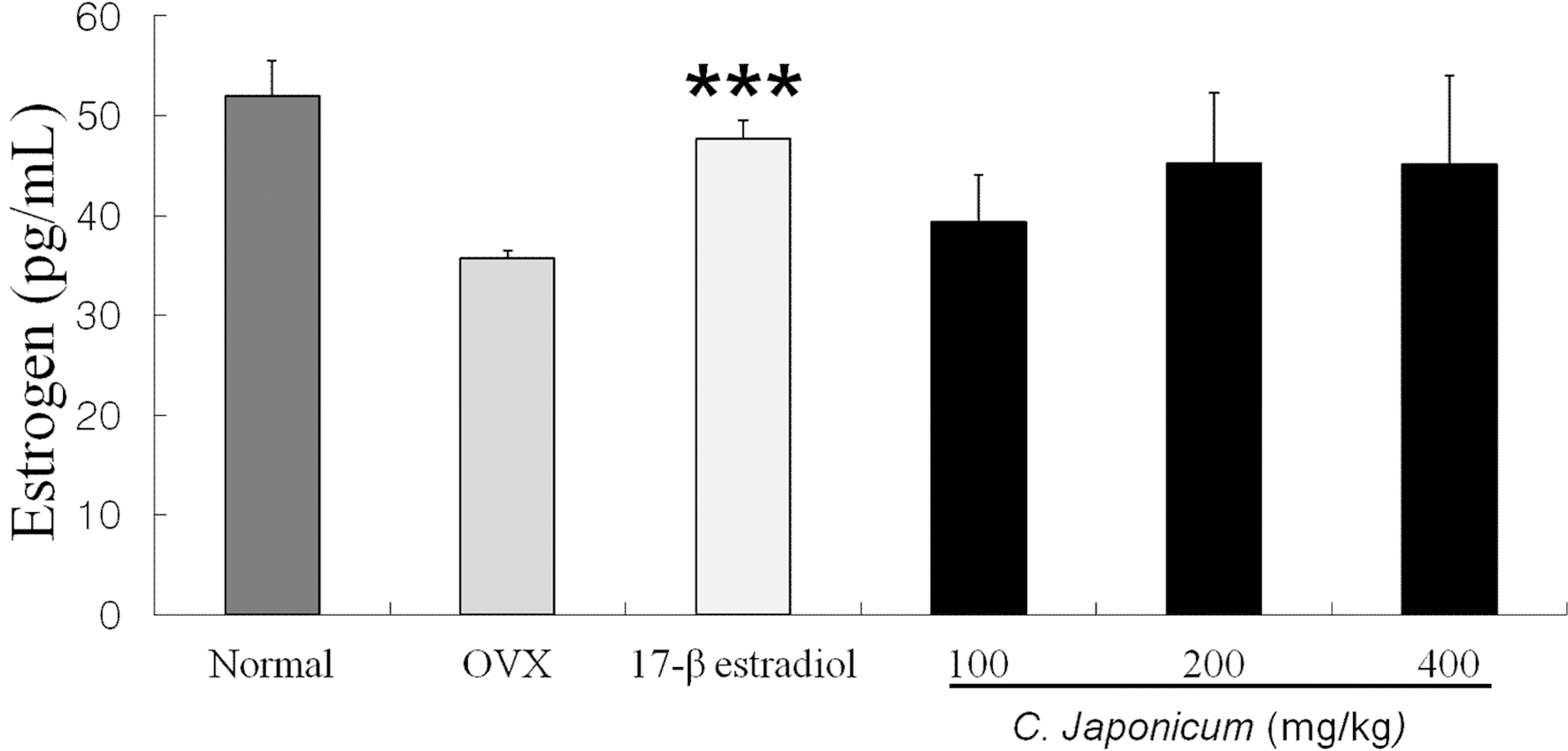
Effects of OVX and C. japonicum on serum estrogen levels determined.Values are mean ± S.E.M. The number of animals is 10/group. Normal; Normal and saline treated group, OVX; ovariectomized and saline treated group OVX+, C. japonicum; ovariectomized and A. japonica treated group. 17-β estradiol; ovariectomized and 17-β estradiol treated group. ***p < 0.001 vs. the OVX group.
5. Effect of ovariectomy and C. japonicum on serum Ca2+ and P levels
Changes in serum level of Ca++ and P after C. japonicum treated group are shown in Fig 6. 7. The serum levels of Ca++ and P in all groups were differences compared with the OVX group (p < 0.05). The serum levels of Ca++ in OVX + C. japonicum (100mg/kg) group (8.35 ± 0.2 ng/mL) was significantly lower than in OVX group (10.04 ± 0.39 ng/mL Fig. 6). On 8 weeks after ovariectomy, serum level of phosphorus on the 17β- estradiol and the 200mg/kg C. japonicum treated group were significantly higher than that of the OVX group (6.96 ± 0.96 ng/mL), but in C. japonicum (400mg/kg) the serum phosphorus decreased to 8.29 ± 0.80 ng/mL.
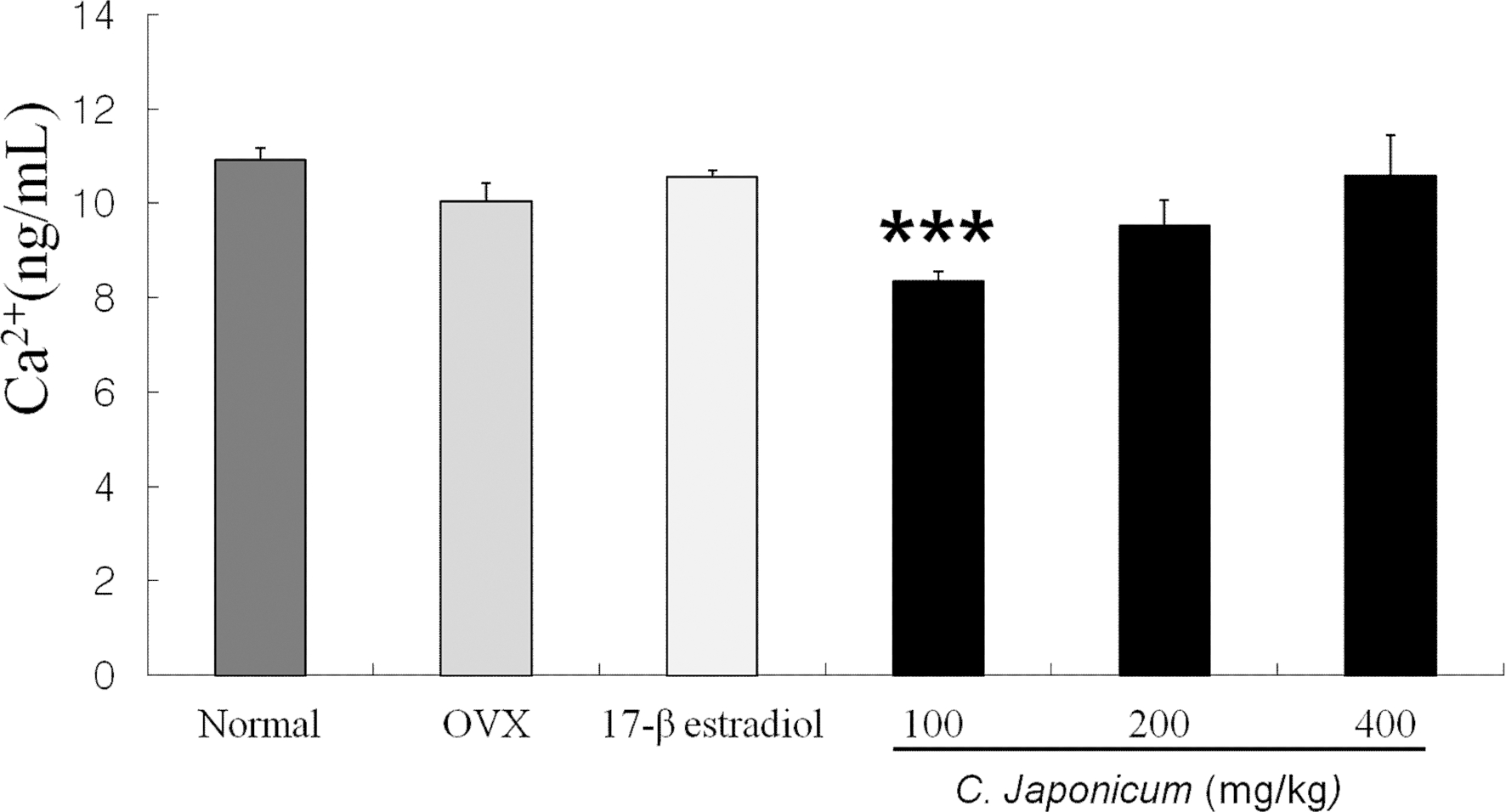
Effects of OVX and C. japonicum on serum Ca2+ levels determined.Values are mean ± S.E.M. The number of animals is 10/group. Normal; Normal and saline treated group, OVX; ovariectomized and saline treated group OVX+, C. japonicum; ovariectomized and C. japonicum treated group. ***p < 0.001 vs. the OVX group.17-β estradiol; ovariectomized and 17-β estradiol (30μg/kg) treated group.
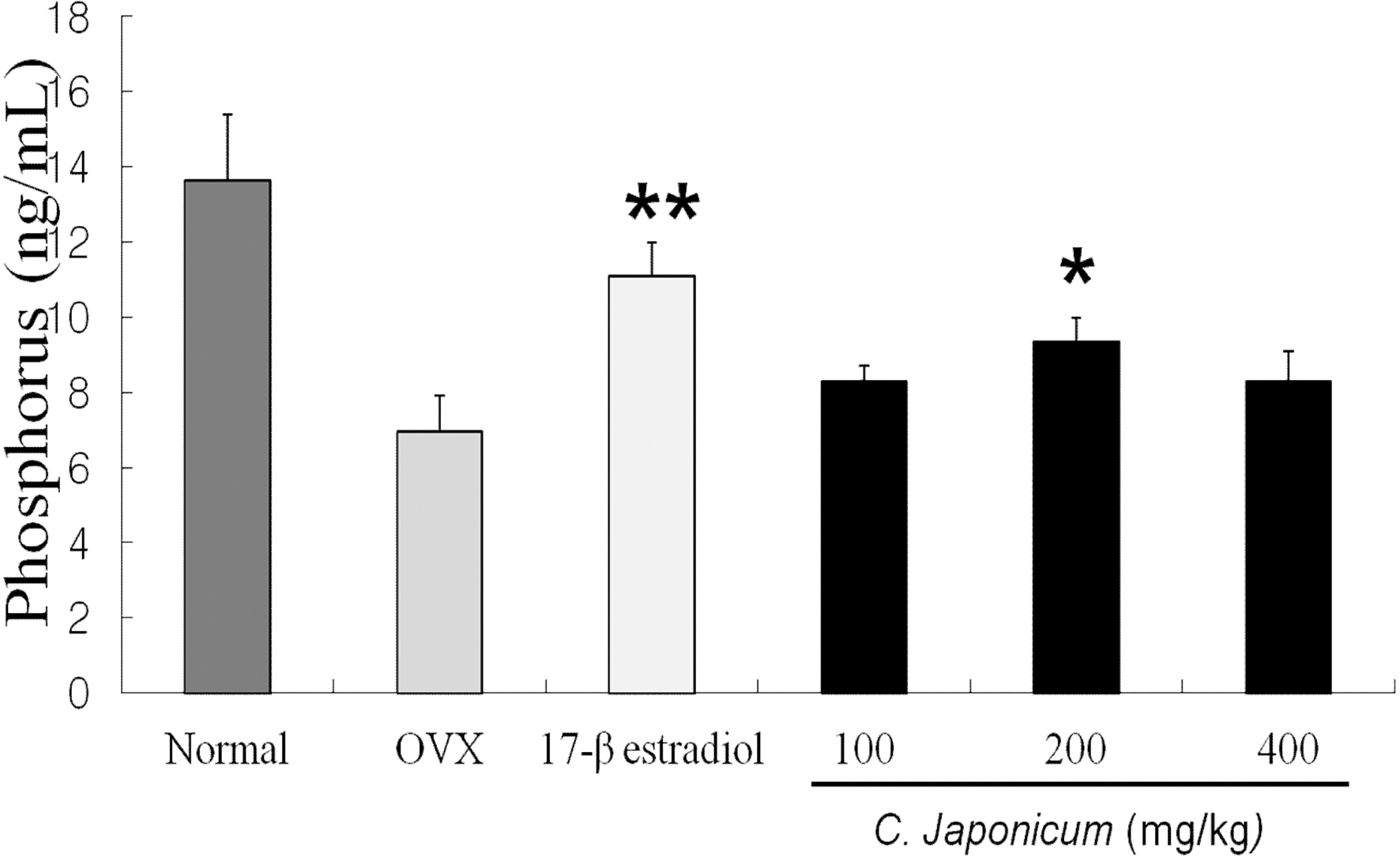
Effects of OVX and C. japonicum on serum phosporus levels determined. Values are mean ± S.E.M. The number of animals is 10/group. Nornal; Normal and saline treated group, OVX; ovariectomized and saline treated group, C. japonicum; ovariectomized and C. japonicum treated group. *p < 0.05 vs. the OVX group.17-β estradiol; ovariectomized and 17-β estradiol treated group. **p < 0.01 vs. the OVX group.
6. Femur / body weight
In Fig. 8, the ratio of femur/body weight on the 17β- estradiol and the C. japonicum (200, 400mg/kg) treated group were significantly higher than that of the OVX group (0.38 ± 0.06 ng/mL).
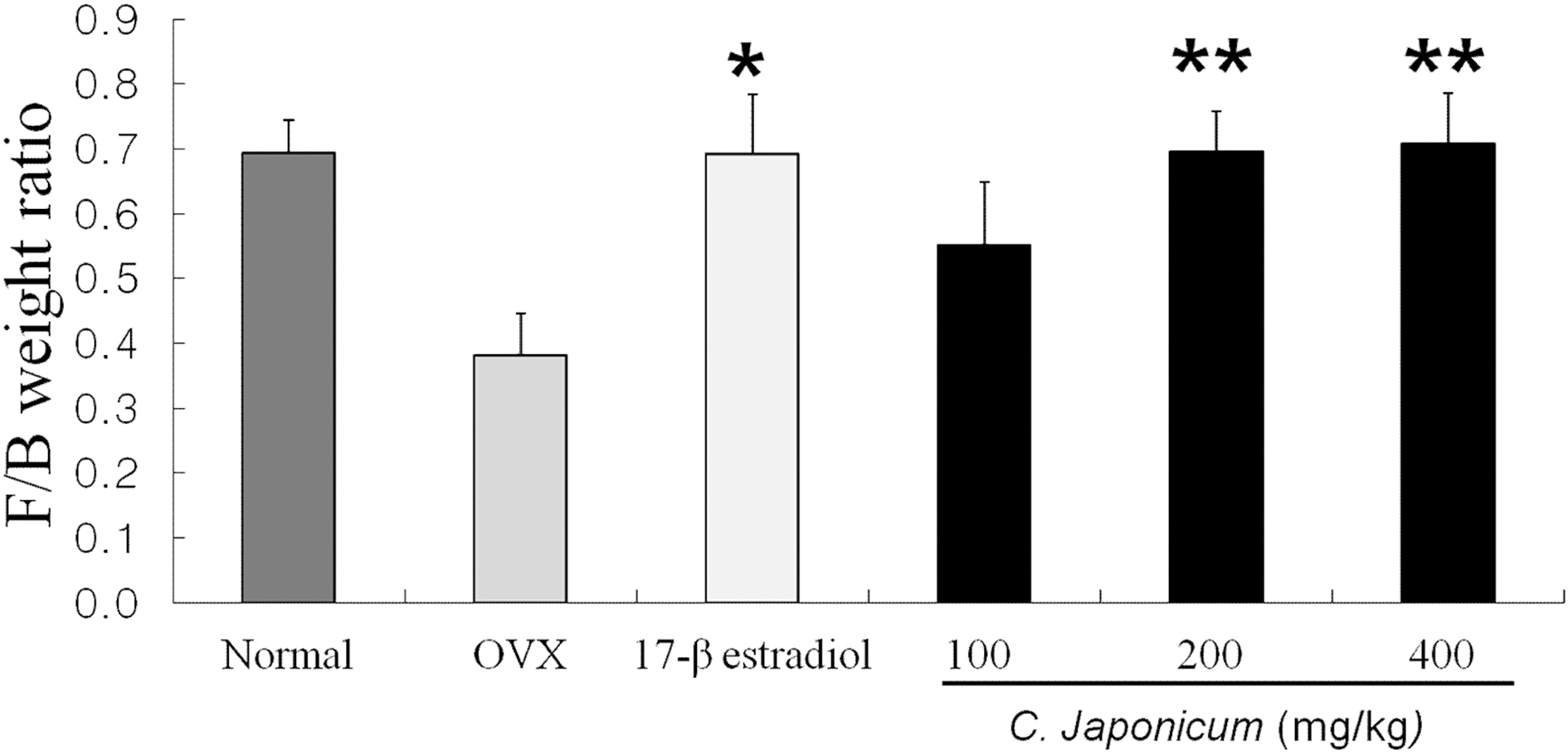
Effects of OVX and C. japonicum on F/B weight ratio levels determined.Values are mean ± S.E.M. The number of animals is 10/group. Normal; Normal and saline treated group, OVX; ovariectomized and saline treated group, C. japonicum; ovariectomized and C. japonicum treated group. **p < 0.01 vs. the OVX group. 17-β estradiol; ovariectomized and 17-β estradiol (30μg/kg) treated group. *p < 0.05 vs. the OVX group.
DISCUSSION
This study showed that the C. japonicum enlargements IGF/IGFBP on osteoporosis induced-ovariectomize rats. OVX dramatically increases body weights, while C. japonicum treatment presents almost normal levels (Fig. 1). Circulating levels of IGF-I, -II is low in men and women with osteoporosis (Frystyk et al., 1994). The growth hormone/IGF-I system stimulates both the bone resorbing and bone-forming cells, but the superior effect is on bone formation, thus resulting in an increase in bone mass. Circulating level of IGF-I and IGFBP-III were also low in males and females with osteoporosis due to decreasing of bone formation or increasing of bone resorption (Nasu et al., 1997). and increasing serum IGF-I levels of ovariectomized rats have been decreased (Rosen et al., 1994). In serum IGF-II levels in C. japonicum treated OVX rats increased in 8 weeks just as osteoblast differentiation and activity aid. IGF-II appears to be due to stimulatory effect of C. japonicum on osteoblast and osteoclast bone formation in this study (Fig. 2, 3).
In this study we investigated IGFBP-III levels to determine the role of IGFBP-III in bone growth. The IGFBP-III levels were high in the C. japonicum group but not significantly different from those of the OVX group. The serum IGFBP-III levels did not exactly reflect the IGF-I level. However, the results indicate that the IGF-1 and IGFBP-III levels had a directly proportional relationship. Most rats with high IGF-1 levels had high IGFBP-III levels as well (Fig. 4). These findings suggest that IGFBP-III also could be a biomarker of bone growth. We studied the increase of IGF-I during C. japonicum treated osteoporosis compared with the OVX group after ovariectomy. IGF-I induces early osteoblast gene expression in human mesenchymal stem cells (Koch et al., 2005) and increased bone remodeling in transgenic mice with osteoblast targeted insulin-like growth factor-I (Jiang et al., 2006).
Estrogen play a major role acts in mineral homeostasis, ‘the deficiency of estrogen is recognized as a major factor in loss of bone minerals in postmenopausal osteoporosis (Danielsen and Flyvbjerg, 1996). IGF-I induces cell proliferation via the action in the granulose cells of the ovary and the Leydig cells of the testis and increases the synthesis of the female hormones (estradiol, progesterone) and the male hormone (testosterone) under the control of gonadotropic hormone (Kim et al., 2003b) (Fig. 5). IGFBP-3, the quantitatively predominant IGFBP in circulation, is positively regulated by growth hormone and potentiates IGF action under most conditions. IGFBP-III has the unique property of being able to associate with an acid-labile subunit (ALS) after IGF binding, thus forming a 150 kd complex (Fig. 6, 7).
Longitudinal bone growth is concern with the expression of various growth hormons (GH). GH induces the IGF-I expression in the liver, and circulating IGF-I in serum stimulates skeletal growth (Yakar et al., 2002) and affects the expression of IGF-I in the bone (Roith et al., 2001). In this study, OVX dramatically increases body weights, while C. japonicum treatment presents almost normal levels. Although the mechanisms by which OVX induces an increase in body weight are not clear, estrogen deficiency induced body fat accumulation and subsequently caused an increase in body weight (Dang et al., 2002) (Fig. 8). Therefore, this study indicates that effects of serum IGFs expression by treated C. japonicum may be related to growth rate in OVX rats. In conclusion, C. japonicum is able to prevent OVX-induced in bone loss, suggesting that C. japonicum may be a reasonable natural alternative for the prevention of postmenopausal osteoporosis.
ACKNOWLEDGEMENTS
This study was financially supported by the Medicinal Crops Division, Ginseng and Medicinal Plants Research Institute, Rural Development Administrations(PJ010221).
REFERENCES
-
Ammann, P, Rizzoli, R, Meyer, JM, Bonjour, JP, Bone density and shape as determinants of bone strength in IGF-I and/or pamidronate-treated ovariecto-mized rats, Osteoporos International, (1996), 6, p219-227.
[https://doi.org/10.1007/bf01622738]

- Baxter, RC, Circulating binding proteins for the insulin like growth factors, Trends in Endocrinology and Metabolism, (1993), 4, p91-96.
-
Binoux, M, Hossenlopp, P, Insulin-like growth factor(IGF) and IGF-binding proteins: Comparison of human serum and lymph, Journal of Clinical Endocrinolgy Metabolism, (1988), 67, p509-14.
[https://doi.org/10.1210/jcem-67-3-509]

- Christiansen, C, Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis, The American Journal of Medicine, (1993), 94, p646-650.
- Chung, HY, Sung, B, Jung, KJ, Zou, Y, Yu, BP, The molecular inflammatory process in aging, Antioxidant & Redox Signaling, (2006), 18, p572-581.
-
Dang, ZC, Van Bezooijen, RL, Karperien, M, Papapoulos, SE, Lowik, CW, Exposure of KS483 cells to estrogen enhances osteogenesis and inhibits adipogenesis, Journal of Bone and Mineral Research, (2002), 17, p394-405.
[https://doi.org/10.1359/jbmr.2002.17.3.394]

- Dinielsen, CC, Flyvbjerg, A, Insulin-like growth Factor I as a predictor of cortical bone mass a long-term study of ovariectomized an estrogen-treated rats, Bone, (1996), 19, p493-498.
- Epstein, S, Epstein update of current therapeutic options for the treatment of postmenopausal osteoporosis, Clinical Therapeutics, (2006), 28, p151-173.
-
Jiang, J, Lichtler, AC, Gronowicz, GA, Transgenic mice with osteoblast targeted insulin-like growth factor-I show increased bone remodeling, Bone, (2006), 39, p494-504.
[https://doi.org/10.1016/j.bone.2006.02.068]

- Jiro, F, Shumpei, N, Yuji, Y, Akira, Y, Mitsuo, O, Wear debris stimulates bone-resorbing factor expression in the fibroblasts and osteoblasts, Hip International, (2011), 21, p231-237.
-
Juul, A, The effects of oestrogens on linear bone growth, Human Reproduction Update, (2001), 7, p303-313.
[https://doi.org/10.1093/humupd/7.3.303]

- Kim, DY, Kang, SH, Ghil, SH, Cirsium japonicumextract induces apoptosis and anti-proliferation in the human breast cancer cell line MCF-7, Molecular Medicine Reports, (2010), 3, p427-432.
- Kim, SH, Ku, SY, Jee, BC, Clinical efficacy of serum insulin-like growth factor-I(IGF-I), IGF-II, and IGF binding protein-3(IGFBP-3) in predicting the prognosis of in vitro fertilization and embryo transfer, Korean Journal of Obstetrics & Gynecology, (003b), 46, p802-809.
-
Koch, H, Jadlowiec, JA, Campbell, PG, Insulin-like growth factor-I induces early osteoblast gene expression in human mesenchymal stem cells, Stem Cells and Development, (2005), 14, p621-631.
[https://doi.org/10.1089/scd.2005.14.621]

- Lee, HK, Kim, JS, Kim, NY, Kim, MJ, Park, SU, Yu, CY, Antioxidant, antimutagenicity and anticancer activities of extracts fromCirsium japonicumvar. ussuriense KITAMURA, Korean Journal of Medicinal Crop Science, (2003), 11, p53-61.
-
McLean, RR, Proinflammatory cytokines and osteoporosis, Current Osteoporosis Reports, (2009), 7, p134-139.
[https://doi.org/10.1007/s11914-009-0023-2]

-
Nasu, M, Sugimoto, T, Chihara, M, Hiraumi, M, Kurimoto, F, Chihara, K, Effect of natural menopause on serum levels of IGF-I and IGF-binding protein : Relationship with bone mineral density and lipid metabolism in perimenopausal woman, European Journal of Endocrinology, (1997), 136, p608-616.
[https://doi.org/10.1530/eje.0.1360608]

- Nilsson, A, Ohlsson, C, Isaksson, OG, Lindahl, A, Isgaard, J, Hormonal regulation of longitudinal bone growth, European Journal of Clinical Nutrition, (1994), 48, p150-158.
-
Park, MK, Rhyu, MR, Yoon, BK, Kwon, H, Jang, S, Lee, YJ, Modulation of the genomic estrogen receptor pathway by water extracts ofCirsium japonicum, Archives of Pharmacal Research, (2008), 31, p225-230.
[https://doi.org/10.1007/s12272-001-1145-y]

-
Park, YC, Lee, JS, Kim, DY, Son, HY, Lee, JW, Cheoi, YS, Kim, KK, Yu, CY, Chung, IM, Im, MH, Lee, KJ, Choi, RN, Shim, HS, Lim, JD, A 90 day repeated dose-oral toxicity study of extracts fromAstragalus membranaceus-aboveground parts in rats, Korean Journal of Medicinal Crop Science, (2013), 21, p474-485.
[https://doi.org/10.7783/kjmcs.2013.21.6.474]

- Roith, DL, Bondy, C, Yakar, S, Liu, JL, Butler, A, The somatomedin hypothesis, Endocrine Reviews, (2001), 22, p53-74.
-
Rosen, CJ, Donahue, LR, Hunter, SJ, Insulin-like growth factors and bone: the osteoporosis connection, Proceedings of the Society for Experimental Biology and Medicine, (1994), 206, p83-102.
[https://doi.org/10.3181/00379727-206-43726]

-
Rosen, CJ, Pollak, M, Circulating IGF-I: New perspectives for a new century, Trends in Endocrinology and Metabolism, (1999), 10, p136-141.
[https://doi.org/10.1016/s1043-2760(98)00126-x]

-
Stewart, CE, Rotwein, P, Growth, differentiation and survival: Multiple physiological functions for insulin-like growth factors, Physiological Reviews, (1996), 76, p1005-1026.
[https://doi.org/10.1152/physrev.1996.76.4.1005]

- Turner, RT, Maran, A, Lotinun, S, Hefferan, T, Evans, GL, Zhang, M, Sibonga, JD, Animal models for osteoporosis, Review of Endocrinology and Metabolism, (2001), 2, p117-127.
-
Yakar, S, Rosen, CJ, Beamer, WG, Circulating levels of IGF-I directly regulate bone growth and density, Journal of Clinical Investigation, (2002), 110, p771-781.
[https://doi.org/10.1172/jci200215463]

-
Yin, J, Heo, SI, Wang, MH, Antioxidant and antidiabetic activities of extracts fromCirsium japonicumroots, Nutrition Research Practice, (2008), 2, p247-251.
[https://doi.org/10.4162/nrp.2008.2.4.247]
