
Clinical Study on Food Safety evaluation of Panax ginseng
†Corresponding author: (Phone) +86-0431-84533304 zlx863@163.com
© The Korean Society of Medicinal Crop Science All rights reserved
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License ( http://creativecommons.org/licenses/by-nc/3.0 ) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
In this study, the clinical safety and toxicology of oral ingestion of supplement capsules containing ginseng radix was investigated in healthy young volunteers. This study was a pilot randomized, double blinded, placebo controlled trial. The healthy volunteers were divided into 6 groups of 20 each (10 males and 10 females). They took the ginseng powder for 35 days (3g/day) for safety evaluation. There were measured general healthy levels such as hematological, biochemical and electrocardiographic parameters. After the first week, besides Korean white ginseng the other treatments led to an significant increase of white blood cells. Korean red ginseng increased UREA (blood urea nitrogen) in healthy volunteers, but it didn’t exceed the range of normal values, and in the subsequent process of treatment there is no effect of elevating UREA. After the three weeks, Korean white ginseng showed relatively low the content of blood glucose and low-density lipoprotein cholesterol. After the five weeks, compared with the other treatments, Korean red ginseng increased white blood cells, platelet distribution width and average volume of platelet. Korean white ginseng decreased low-density lipoprotein cholesterol. American ginseng decreased blood creatinine in healthy volunteers. In conclusion, through test the blood routine, urine routine, liver function, renal function, blood glucose, blood lipid and electrocardiogram, the healthy volunteers continuous taking ginseng for 35 days (3 g/day) is safe and reliable, and have no obvious adverse reactions and side effects.
Keywords:
Panax ginseng, Panax quinquefolium, Food SafetyINTRODUCTION
Korean ginseng (Panax ginseng C. A. Meyer) has been widely used as medicinal crop for thousands of years because of its quality and efficacy superiority. Korean ginseng have many efficacies such as fatigue recovery, boosting of immune system, improvement of blood circulation, memory improvement, anti-oxidative activity and so on.
Korean ginseng has been used mainly to make medicine for a long time. It is also a good ingredient for cooking diverse foods. At the 27th meeting of the Codex Committee on Processed Fruits and Vegetables of the Codex Alimentarius Commission, the Korean proposal for updating global standards about ginseng products passed. Once the motion receives the approval of the CODEX general assembly, which will convene in Switzerland in 2015, world standards will define ginseng not as a medicinal product but as a food product, making it easier to export. With ginseng products being defined as food instead of medicine, the doors have been opened to be exported to elsewhere around the world.
In Korea, ginseng (P. ginseng) is commonly used in the form of decoction, extract and powder, ginseng products. It is the widely used as food recently. In spite of the importance as food, the study on the safety of ginseng taking is insufficient in comparison with the other studies about ginseng (Han et al., 2013; Kim et al., 2014). In China, which is the largest importer of Korean ginseng, it is inadequate particularly background information on toxicological evaluation of ginseng radix. This could be the chief obstacle for the export expansion of Korean ginseng to the whole world including China. Therefore, foreigners need to be satisfied of the food safety for Korean ginseng.
We conducted collaboration research to resolve this issue with Jilin Agricultural University of China. The aim of this study was to evaluate the safety and toxicology of ginseng radix (P. ginseng, P. quinquefolium) delivered in capsule format in a randomized, double-blind, placebocontrolled study of closely monitored human subjects. The subject of experiment was Chinese young people. The safety of Korean ginseng for them will contribute to consumer layer and demand expand of Korean ginseng in China. Included result was sequential documentation of the clinical hematological, biochemical and electrocardiographic parameters of the treated subjects to determine whether the values obtained extended beyond normal limits or differed significantly from the findings in the placebo group (Mitchell et al., 2012).
MATERIALS AND METHODS
1. Study design
The study was a double-blind, placebo-controlled study consisting of 5 weeks intervention. 120 healthy young volunteers of China (age range 21~25 years) were selected for this study. Before treatment, pulse rate and blood pressure readings were also taken, and a blood sample was taken for clinical examination and laboratory (hematology and blood biochemistry) investigations as well as urine analysis and electrocardiogram. The dosage volume in this study was decided to 3g/day. The average dosage of ginseng is considered to be three to four gram per day unless prescribed apart. It was referred to the data of Korea, China, Japan and USA (Ji and Lee, 1987; Nam and Park, 2000). The study outcome measures were also evaluated at the end of 1 week, 3 weeks and 5 weeks of treatment. All participants provided written informed consent prior to the start of the study. This study was done at Jilin Agricultural University and its accidental Hospital.
2. Volunteers
Volunteers were divided into 6 groups of 20 each (10 males and 10 females). Groups 1 received 3 g 4 years of Chinese white ginseng capsules, group 2 received 3 g 6 years of Chinese red ginseng capsules, group 3 received 3 g 4 years of Korean white ginseng capsules, group 4 received 3 g 6 years of Korean red ginseng capsules, group 5 received 3 g 4 years of American white ginseng capsules, group 6 received placebo (starch flour 3g), respectively, for 35 days. They took the capsule (ginseng powder) everyday on an empty stomach respectively.
3. Materials
4 years of Chinese white ginseng (P. ginseng, produced in China), 6 years of Chinese red ginseng (P. ginseng, produced in China) and 4 years of American ginseng (P. quinquefolium) were purchased from Ji’an city of Jilin Province. 4 years of Korean white ginseng (P. ginseng, produced in Korea) and 6 years of Korean red ginseng (P. ginseng, produced in Korea) were provided from Department of Herbal Crop Research, National Institute of Horticultural Herbal Science, RDA. They were formulated as capsules which each capsule contained 0.25 g dried raw material.
4. Inclusion criteria
Healthy young volunteers and willing to give voluntary written informed consent were selected to take part in this study. The volunteers were proven to be healthy through clinical examination by the physician along with hematological and biochemical investigations as well as electrocardiographic.
5. Exclusion criteria
Volunteers were excluded from this study if any of the following criteria applied at the time of study 1-history of allergy to ginseng radix, 2-history of cardiovascular disease including vascular or conductive cardiac disease, hypertension, 3-history of renal disorder, 4-history of endocrine disease for example: diabetic mellitus, hyperlipidemia, hypercholestrolemia, 5-history of liver disease, 6-volunteers who have taken any drugs.
6. Statistical analysis
Statistical analysis was done using Student’s paired t-test with SPSS software. p-Values less than 0.05 were considered to be statistically significant.
RESULTS AND DISCUSSIONS
Through clinical examination showed no gross changes in clinical sign and symptoms in all volunteers after intervention. No significant differences were noted in the mean values of body weight, temperature, sitting systolic and diastolic blood pressure as well as electrocardiographic. Parameters of a routine urine test and liver function in six groups before and after the intervention was not changed. The volunteers for the experiment took the ginseng (3 g/ day) of powder form during 35 days. The dosage was determined using the data of several countries (Nam and Park, 2000). We examined the blood routine, urine routine, liver function, renal function, blood glucose, blood lipid and electrocardiogram of volunteers. In the different period of treatment, it had a certain effect on healthy volunteers, the part of treatment enhanced the immune functions.
At the end of 1 week administration, through a routine blood test, compared to the placebo group, American ginseng group increased significantly (p < 0.01) the white blood cells in healthy volunteers, 4 years of Chinese white ginseng, 6 years of Chinese red ginseng, and 6 years of Korean red ginseng also increased white blood cells (p < 0.05). 4 years of Korean white ginseng had no effect on white blood cells (p > 0.05). Compared to the placebo group, 6 years of Chinese red ginseng and American ginseng increased absolute lymphocyte count (p < 0.05). A lymphocyte is a vertebrate’s immune system. A general increase in the number of lymphocytes is known as lymphocytosis, whereas a decrease is known as lymphocytopenia. 4 years of Chinese white ginseng increased neutrophil counts(p < 0.05)(Fig. 1). Absolute neutrophil count is a measure of the number of neutrophil granulocytes present in the blood. Neutrophils are a type of white blood cell that fights against infection (Al-Gwaiz and Babay, 2007). Through blood glucose and blood lipid examination, at the end of 1 week administration, compared to the placebo, 6 years of Korean red ginseng increased UREA (blood urea nitrogen) in healthy volunteers (p < 0.05) (Fig. 1), but it didn’t exceed the range of normal values, and in the subsequent process of treatment there is no effect of elevating blood urea nitrogen. Blood urea nitrogen (BUN) is an indication of renal health. The main causes of an increase in BUN are high protein diet, decrease in glomerular filtration rate and in blood volume, congestive heart failure, gastrointestinal hemorrhage, fever and increase catabolism. The main causes of a decrease in BUN are severe liver disease, anabolic state, and syndrome of inappropriate antidiuretic hormone (Fauci, 2011).
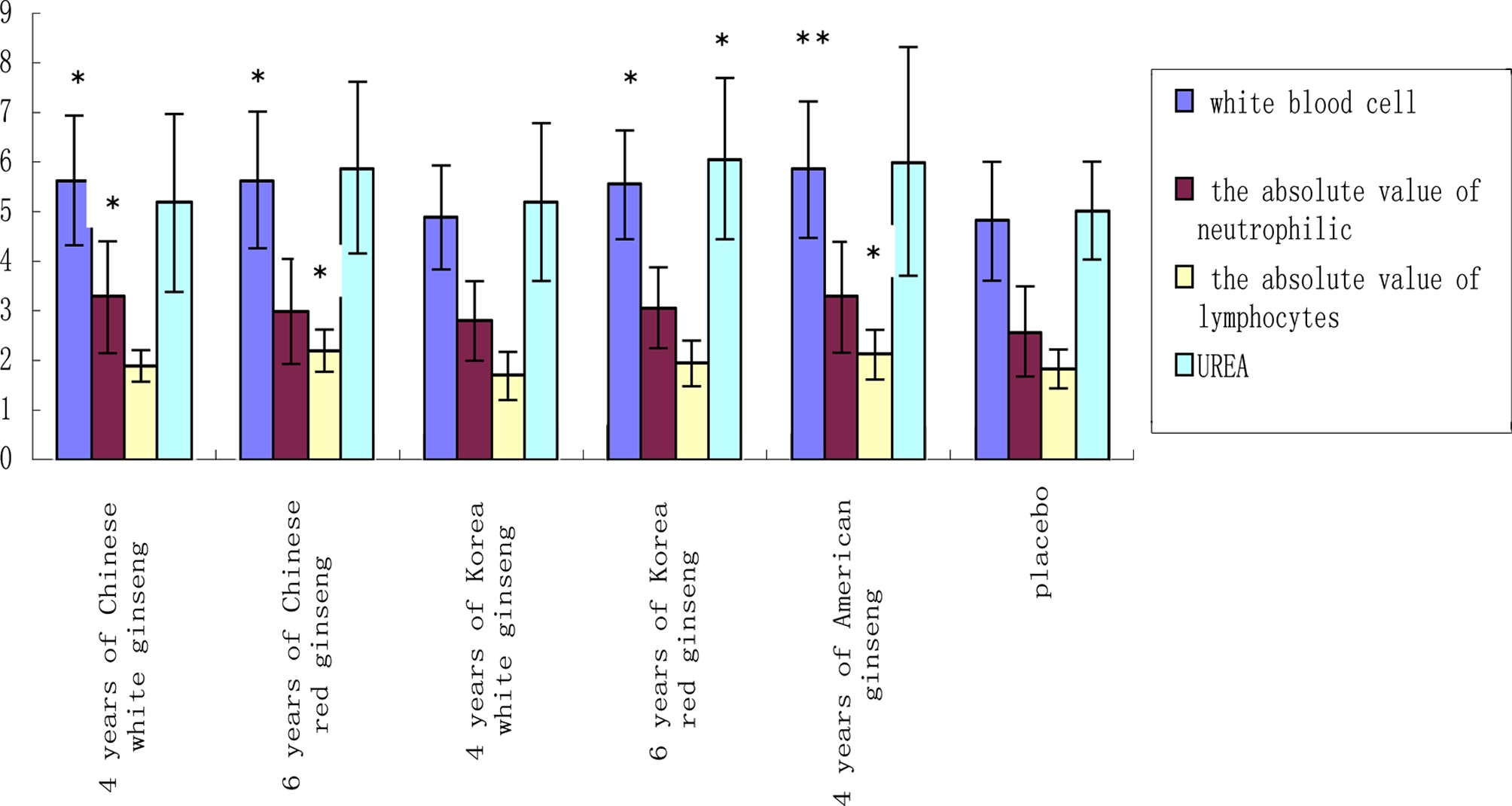
Comparison of each group’s white blood cells (unit: 109/ ℓ ), absolute lymphocyte count (109/ ℓ ), neutrophil counts (109/ ℓ ) and blood urea nitrogen (mmol/ ℓ ) at the end of 1 week administration.The measured value presented as the mean ± SD. Statistical difference (*, p < 0.05; **, p < 0.01).
At the end of 3 weeks administration, through a routine blood test, compared to the placebo group, 4 years of Chinese white ginseng increased white blood cells (p < 0.05). While the other group have no any effect on white blood cells (p > 0.05). Through blood glucose and blood lipid examination, at the end of 3 weeks administration, compared to the placebo, 4 years of Korean white ginseng decreased the blood glucose (GLUC) and low-density lipoprotein cholesterol (LDLC) in healthy volunteers (p < 0.05) (Fig. 2).Blood glucose level is the amount of glucose (sugar) present in the blood of a human or animal. The body naturally tightly regulates blood glucose levels as a part of metabolic homeostasis (Mark et al., 1998). LDLC is an important marker when it comes to assessing the risk for developing heart disease. LDL -particles, in particular those who are small and dense, are atherogenic and increase the risk of cardiovascular disease.
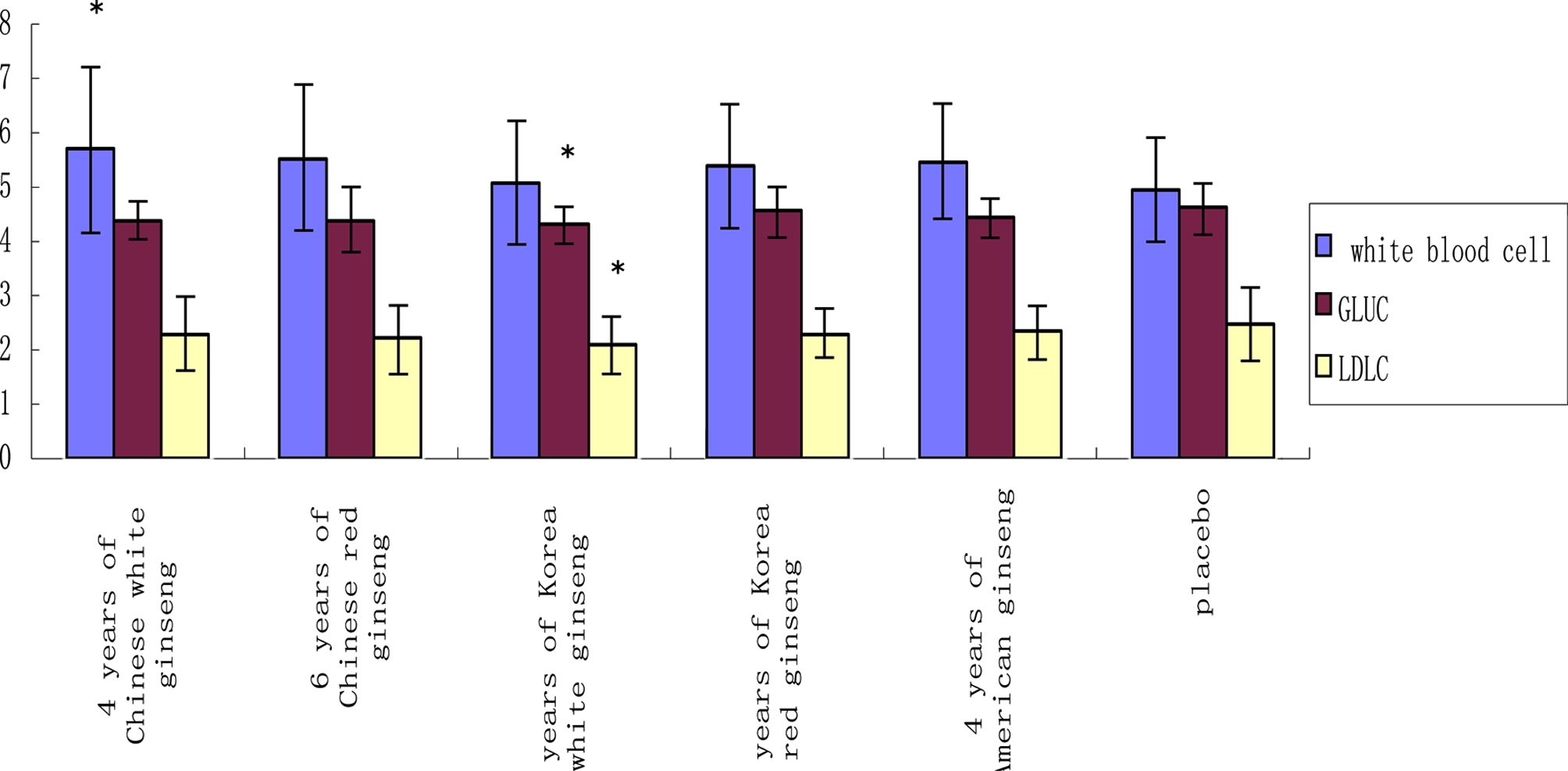
Comparison of each group’s white blood cell (unit: 109/ ℓ ), GLUC (mmol/ ℓ ) and LDLC (mmol/ ℓ ) at the end of 3 weeks administration.GLUC; blood glucose, LDLC; low-density lipoprotein cholestrol. The measured value presented as the mean ± SD. Statistical difference (*, p < 0.05).
Through a routine blood test, at the end of 5 weeks administration, compared to the placebo group, 6 years of Korean red ginseng increased white blood cells, platelet distribution width (PDW) and average volume of platelet (PDV) (p < 0.05). While the other group have no any effect on them (Fig. 3). Platelets are blood cells whose function (along with the coagulation factors) is to stop bleeding. This bleeding can be caused by deficient numbers of platelets, dysfunctional platelets, or very excessive numbers of platelets (Laki, 1972; Murakawa et al., 1992). 4 years of Korea white ginseng decreased LDLC in healthy volunteers (p < 0.05) (Fig.3). Through blood glucose and blood lipid examination, at the end of 5 weeks administration, compared to the placebo, American ginseng decreased blood creatinine in healthy volunteers (p < 0.05) (Fig. 4). Creatine is a nitrogenous organic acid that occurs naturally in vertebrates and helps to supply energy to all cells in the body. The liver and kidney contain approximately 0.01% creatine.
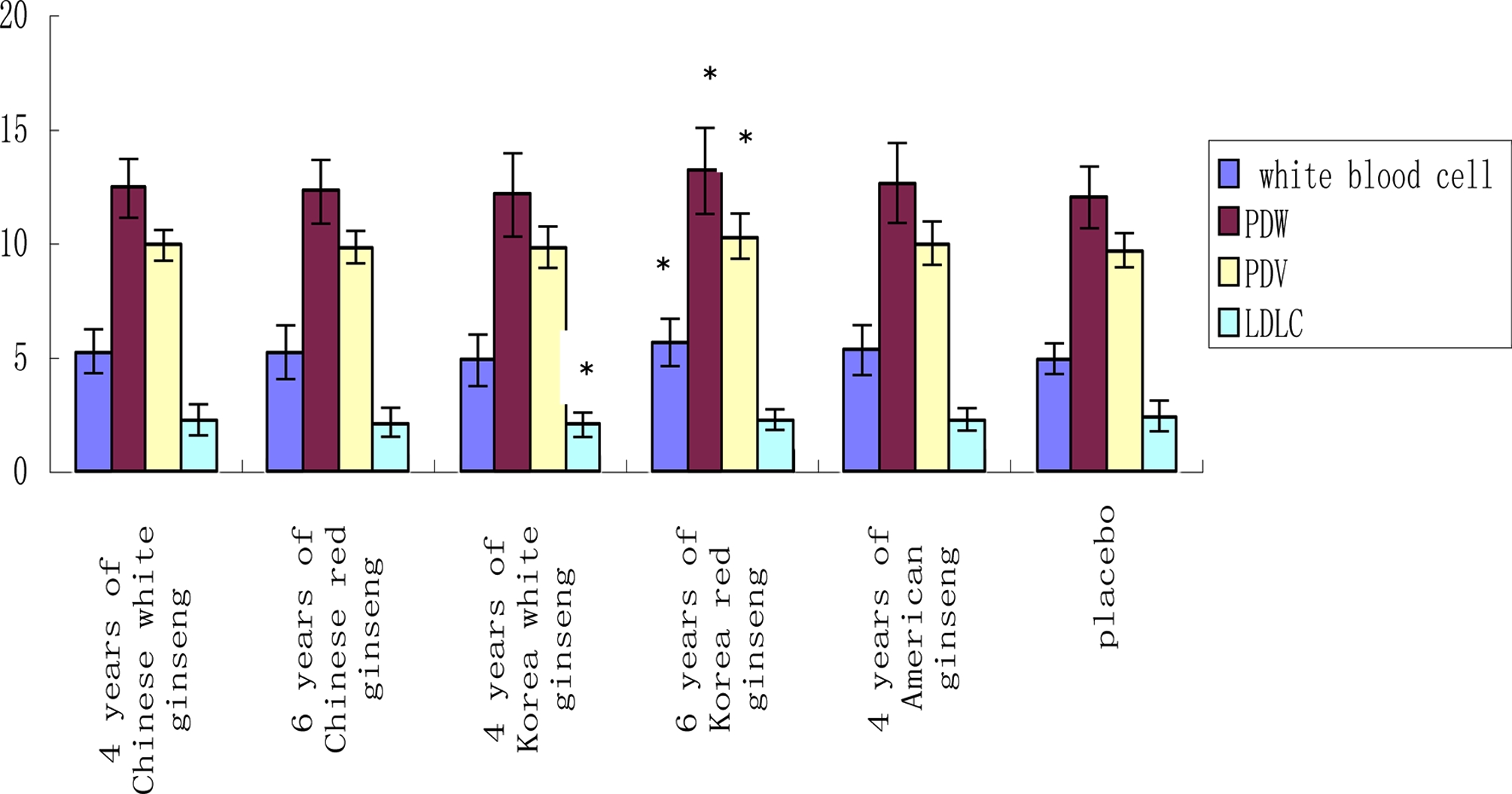
Comparison of each group’s white blood cell (unit: 109/ ℓ ), PDW (1012mℓ), PDV (1012/mℓ) and LDLC (mmol/ ℓ ) at the end of 5 weeks administration.PDW; platelet distribution width, PDV; average volume of platelet, LDLC; low-density lipoprotein cholesterol. The measured value presented as the mean ± SD. Statistical difference (*, p < 0.05).
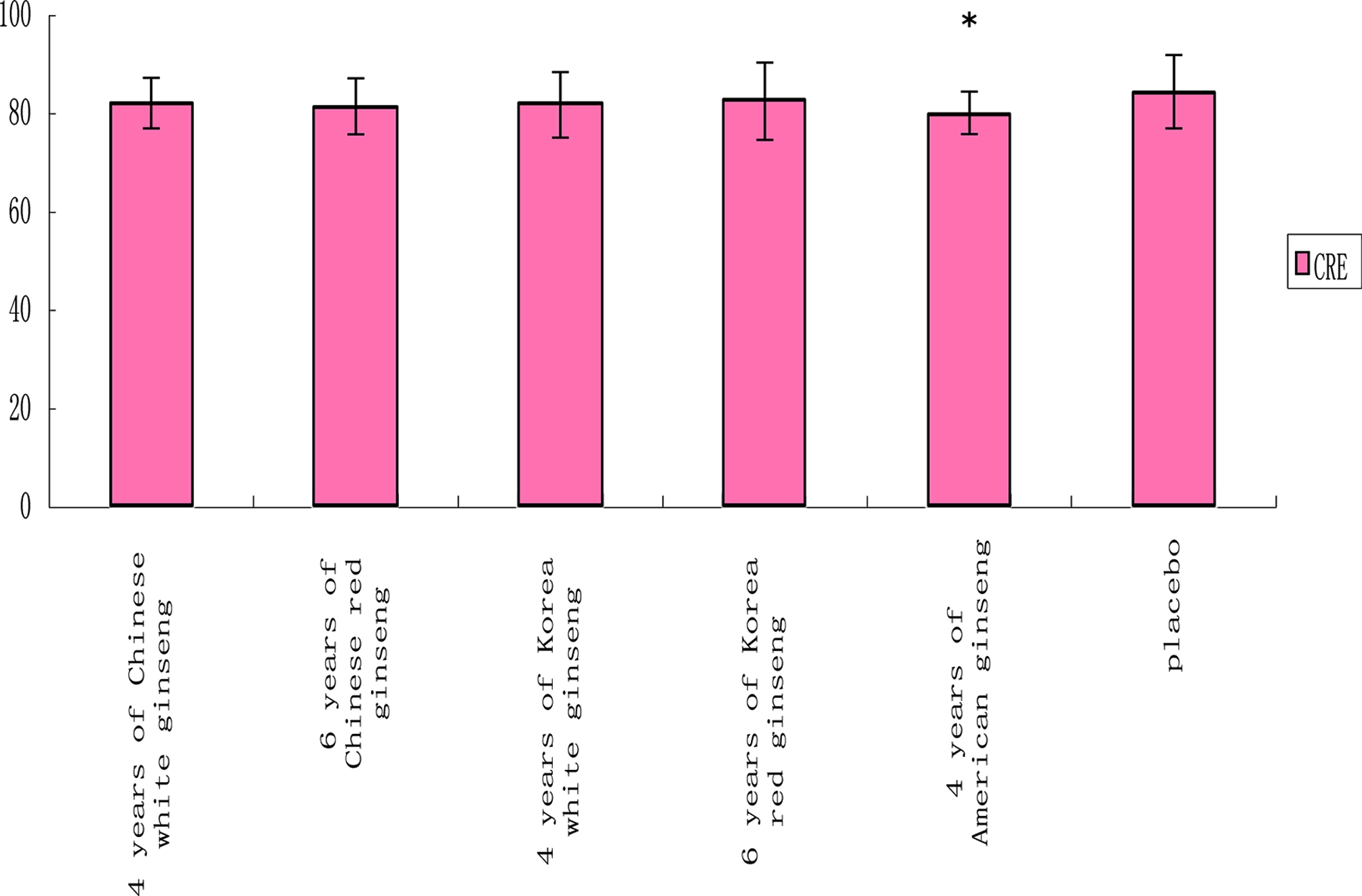
Comparison of each group’s CRE (μmol/ ℓ ) at the end of 5 weeks administration. CRE; blood creatine.The measured value presented as the mean ± SD. Statistical difference (*, p < 0.05).
Many researchers have found that Korean ginseng has the excellent efficacy in human. Recently Korean ginseng is the widely used as medicine, health functional food and food in Korea (Lee et al., 2005; Park et al., 2007). However, the study on the food safety of Korean ginseng is not many all over the world. This study conducted to propagate around the world including China the safety as food of Korean ginseng. A once daily dose of different ginseng radix in capsule format taken over 5 weeks was used to demonstrate safety and toxicology in healthy young volunteers. In conclusion, through test the blood routine, urine routine, liver function, renal function, blood glucose, blood lipid and electrocardiogram, indicated that healthy volunteers continuous taking ginseng for 35 days (3 g/day) is safe and reliable, and have no obvious adverse reactions and side effects. This study also showed that ginseng radix may change some hematological and biochemical parameters. However, these alterations were in normal ranges and they were not important clinically. Above this, we examined many items during the trial period, which are total cholesterol, triglyceride, high density lipoprotein cholesterol, direct bilirubin, total bilirubin, mean corpuscular hemoglobin, mean corpuscular, red cell distribution width standard deviation, alkaline phosphatase, gamma-glutamyl transpeptidase and carbon dioxide combining power, and there was no significant difference between the treatments (It was not given the data.). All the result of this study prove the food safety of Korean ginseng.
ACKNOWLEDGMENTS
The research was carried out with the support of Cooperative Research Program for Agriculture & Technology Development(PJ00633206) Rural Development Administration, Republic of Korea. We are thankful to College of Chinese Medicinal Materials and accidental hospital of Jilin Agricultural University.
REFERENCES
-
Al-Gwaiz, LA, Babay, HH, The diagnostic value of absolute neutrophil count, band count and morphologic changes of neutrophils in predicting bacterial infections, Medical Principles and Practice, (2007), 16, p344-347.
[https://doi.org/10.1159/000104806]

- Fauci, AS, Harrison's principles of internal medicine, (2011), (18th ed.), p611.
- Han, JS, Tak, HS, Lee, GS, Kim, JS, Ra, JW, Choi, JE, Comparison of ginsenoside content and ratio of root tissue according to root age and diameter in Panax ginseng, Korean Journal of Medicinal Crop Science, (2013), 21, p342-347.
- Ji, HJ, Lee, SI, The Korean herbal pharmacopoeia of the Korean pharmacopoeia, Korean Medical Index, (1987), -636.
- Kim, YC, Kim, YB, Park, HW, Bang, KH, Kim, JU, Cho, IH, Kim, KH, Song, BH, Kim, DH, Optimal harvesting time of ginseng seeds and effect of gibberellic acid(GA3) treatment for improving stratification rate ofginseng(Panax ginseng C. A. Meyer) seeds, Korean Journal of Medicinal Crop Science, (2014), 22, p423-428.
-
Laki, K, Our ancient heritage in blood clotting and some of its consequences, Annals of the New York Academy of Sciences, (1972), 202, p297-307.
[https://doi.org/10.1111/j.1749-6632.1972.tb16342.x]

- Lee, JW, Do, JH, Market trend of health functional food and the prospect of ginseng market, Journal of Ginseng Research, (2005), 29, p206-214.
-
Daly, ME, Vale, C, Walker, M, Littlefield, A, Alberti, KG, Mathers, JC, Acute effects on insulin sensitivity and diurnal metabolic profiles of a high-sucrose compared with a high starch diet, American Journal of Clinical Nutrition, (1998), 67, p1186-1196.
[https://doi.org/10.1093/ajcn/67.6.1186]

-
Mitchell, LJ, Christopher, JM, Erminia, DP, Ryan, RS, Satya, PA, Evaluation of clinical safety and tolerance of a Lactobacillus reuteri NCIMB 30242 supplement capsule: A randomized control trial, Regulatory Toxicology and Pharmacology, (2012), 63, p313-320.
[https://doi.org/10.1016/j.yrtph.2012.04.003]

-
Murakawa, M, Okamura, T, Tsutsumi, K, Tanoguchi, S, Kamura, T, Shibuya, T, Harada, M, Niho, Y, Acquired von Willebrand's disease in association with essential thrombocythemia: Regression following treatment, Acta Haematologica, (1992), 87, p83-87.
[https://doi.org/10.1159/000204725]

- Nam, KY, Park, JD, Usage and dosage of ginseng radix based upon traditional and recent scientific clinical applications, Journal of Ginseng Research, (2000), 24, p99-105.
- Park, CK, Kwak, YS, Hwang, MS, Kim, SC, Do, JH, Current status of ginseng products in market of health functional food, The Korean Ginseng Research and Industry, (2007), 1, p9-16.