Attenuation Effect of Chamaecyparis obtusa Leaf Essential Oils on Airway Hyperresponsiveness and Airway Inflammation in Ovalbumin-Induced Murine Asthma Model
© The Korean Society of Medicinal Crop Science All rights reserved
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License ( http://creativecommons.org/licenses/by-nc/3.0 ) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
In this study, essential oils were extracted from the leaf of Chamaecyparis obtusa (CLEO), indigenous to Korea, CLEO constituents were analysed, and the effects of CLEO on airway hyperresponsiveness (AHR) and airway inflammation (AI) were investigated in Ovalbumin (OVA)-induced asthma mouse model. Terpenoid components among identified CLEO constituents made up more than 80%. The CLEO-treated group in comparison to the control group showed reduced AHR, the decrease of eosinophil number in the bronchoalveolar lavage fluid (BALF), reduced specific anti- OVA IgE level in the serum, and a significant reduction in Th2 cytokines levels in the BALF with concentration. We concluded that CLEO have an alleviating effect on asthma-like symptoms such as AHR and AI. Further studies about antiasthmatic effect are necessary on the focus of single component of CLEO.
Keywords:
Chamaecyparis obtusa, Airway Inflammation, Airway Hyperresponsiveness, Asthma, Leaf Essential OilINTRODUCTION
Asthma is an allergic disease that affects approximately 300 million people worldwide, and it has become a global health concern as its social and economic burdens have risen with its prevalence in recent years (Bateman et al., 2008). Asthma is a chronic disease characterized by airway hyperresponsiveness (AHR), eosinophilic airway inflammation, and reversible airway obstruction, which involves many kinds of cells and various mediators. The chronic inflammation that is associated with AHR can lead to clinical symptoms, such as wheezing, breathlessness, chest tightness, and coughing (Miller, 2001; Holgate, 2008). In addition, unique asthma-induced histological and structural changes include hypertrophy and hyperplasia of airway smooth muscle cells, goblet cell hyperplasia, and subepithelial fibrosis, etc., which have been associated with airway inflammatory reactions (James et al., 1989; Nelson et al., 2003).
Most of respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD) are a chronic disease that require continued treatment, which involves management of the environment and drug treatments. However, the medications have been reported to produce side effects such as hypertension, osteoporosis, headaches, and nausea with prolonged use (Bateman et al., 2008; Pyun, 2007). Reports indicate that long-acting β-agonist can worsen severe asthma cases and increase the risk of asthma-related death (Spitzer et al., 1992; Salpeter et al., 2006). Therefore, recent studies have actively explored natural substances that can reduce these side effects and increase the effectiveness of chronic respiratory diseases prevention and treatment (Kim et al., 2014; Kwak andLim, 2013; Lim and Kim, 2012; Lee et al., 2011).
Chamaecyparis obtusa is an evergreen belonging to the Cupressaceae family. It is native to Japan, and in Korea, and it grows mostly on Jeju Island and in southern regions. C. obtusa can grow up to 40m high and 2m in diameter, and has ovate leaves that are 11.5mm in length and reddish brown bark that splits vertically. Previous studies on the use of C. obtusa essential oil have reported antibacterial effects in the gram-positive bacterium Staphylococcus epidermidis (the gram-negative bacteria Pseudomonas putida, Vibrio parahaemolicus) and Pseudomonas aeruginosa (the filamentous fungi Aspergillus nidulas, Alternaria mali) and Fusarium oxysporum and the yeast Candida albicans (Lee et al., 2001), deodorization effect (Kim et al., 2009), and whitening and anti-oxidant (Kim et al., 2011). In antiinflammatory research on C. obtusa, C. obtusa leaf extract had an effect on immune function in the skin of NC/Nga mice that were used as an atopic dermatitis model (Cho, 2012). Also, C. obtusa essential oil, acting through the cyclooxygenase-2 (COX-2) pathway, was able to control the gene expression of prostaglandin E2 (PGE2) and tumor necrosis factor-α (TNF-α) to produce anti-inflammatory effects on lipopolysaccharide (LPS)-induced inflammation in rats (An et al., 2013). These anti-inflammatory effects of C. obtusa essential oil suggest its potential to mitigate asthma through inhibition of the inflammatory response. Therefore, in this study, we extracted the essential oils from the leaf of Chamaecyparis obtusa, indigenous to Korea, and analyzed the components of its essential oils (CLEO), and investigated immunologically the effects of CLEO against airway hyperresponsiveness (AHR) and airway inflammation (AI) by using asthma mouse model.
MATERIALS AND METHODS
1. Chamaecyparis obtusa leaf essential oil (CLEO) preparation
C. obtusa leaves were collected in Yeongdong-gun Jagye-ri, Chungchoengbukdo, Korea and dried under shade at room temperature for 23 days after washing with distilled water. Essential oil was extracted using a simultaneous steam distillation and extraction (SDE) apparatus (Serkan and Gonca, 2009). One hundred grams of blended C. obtusa leaves was mixed with 1,000 mL of distilled water in a round bottom flask, and the flask was attached to the arm of the SDE apparatus. Pentane from Junsei (purity 99.0%, Japan) and diethyl ether from Merk (purity 99.7%, Germany) were used as solvents and mixed in a round bottom flask (1 : 1, v/v) that was attached to the other arm of the SDE apparatus. The distillationextraction was continued for 3 h. The extract was dried with Na2SO4 (Junsei, Japan) overnight and concentrated by N2 gas. By repeating this process, approximately 83 mL of essential oil was extracted from 4kg of C. obtusa leaves.
2. Analysis of CLEO constitruents.
The gas chromatography-mass spectrometry (GC-MS) analysis of CLEO was performed using an Agilent 6890N GC (Agilent Technologies, Santa Clara, CA, USA) interfaced with an Agilent 5975 (Agilent Technologies, Santa Clara, CA, USA) inert mass selective detector. The GC column was a HP-INOWAX (60 m × 0.25mm id, 0.25μm film thickness) capillary column (Agilent J&W, Agilent Technologies, Santa Clara, CA, USA). The carrier gas was helium with a flow rate of 1 mL/min and sample (0.2 μL) was injected. Inlet temperature was 250°C and oven temperature was programmed to rise from 40 to 240°C at rate of 8°C/min. MS were taken at 70 eV and electron scanning ranges was 15500 amu (Serkan and Gonca, 2009). The essential oil components were identified by matching their recorded mass spectra with the Wiley/7n mass spectral database (Hewlett-Packard CO., Palo Alto, CA, USA).
Among the constituents of CLEO, quantitative analysis of components that were commercially available as reference materials (such as α-pinene, limonene, bornyl acetate, and terpinene-4-ol) was performed. The reference materials were purchased from Sigma Aldrich Co. (St Louis, MO, USA). The GC-MS analysis conditions were identical to those of the qualitative analysis, and the validation method verified the precision, accuracy, and linearity of the calibration curve. The standard material used to create the calibration curve was prepared by dilution in methanol.
3. Animals and breeding condition
Six-week-old female BALB mice (21 ± 1 g) were obtained from Daehan Biolink Co. of Seongnam, Korea. All animals were given solid feed (crude protein ≥ 22.1%, crude fat ≤ 8.0%, crude fiber ≤ 5.0%, crude ash ≤ 8.9%, calcium ≥ 0.6%, and phosphorus ≥ 0.4%) in the form of a standard laboratory diet (Samyang feed, Seoul, Korea) and tap water as libitum. The room was maintained at a temperature of 20 ± 2°C with relative humidity of 50 ± 10%, and a 12 h/12 h light/dark cycle. The experimental animal protocol was approved by the Institutional Animal Care and Use Committee of Deajeon University.
4. OVA-induced asthma mouse model
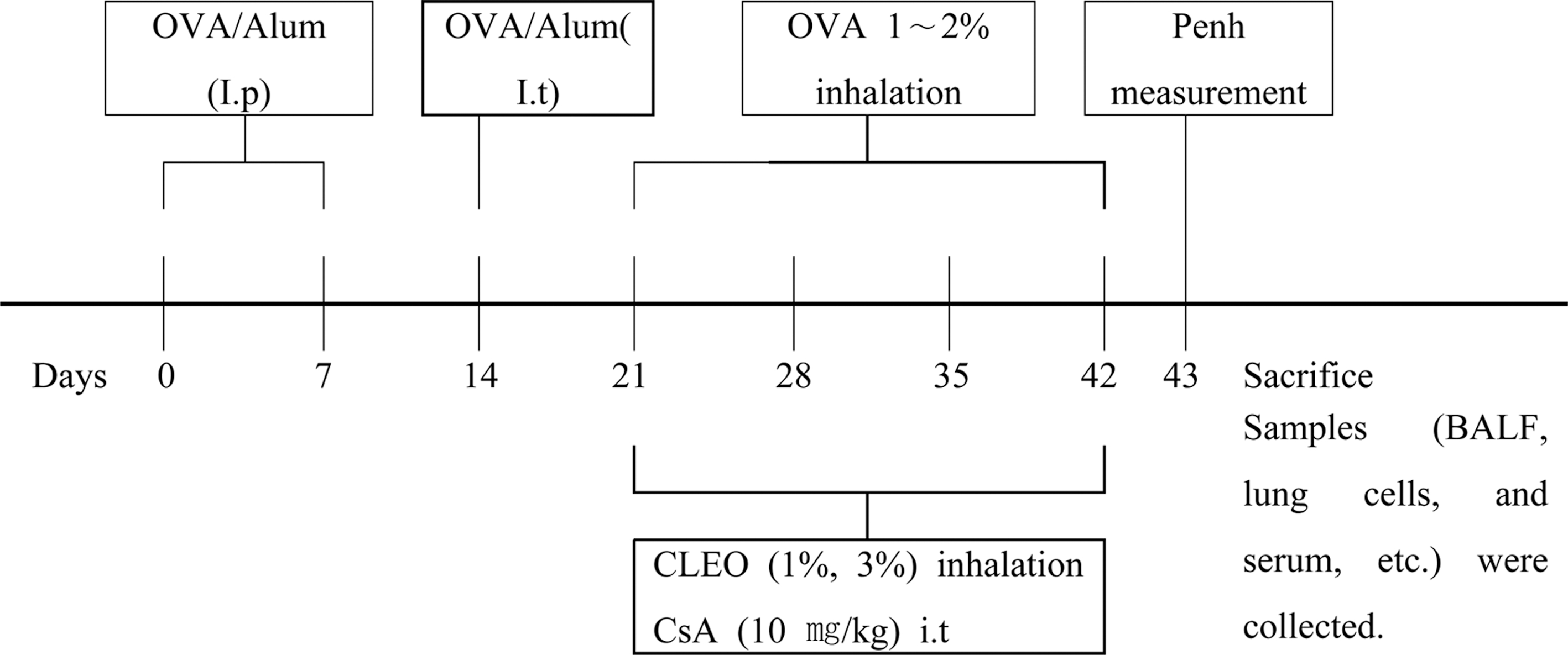
The OVA-induced asthma mouse model method was modified from Lim and Kim (2009). OVA (500μg/mL) in phosphate buffer saline (PBS) was mixed with an equal volume of 10% (w/v) aluminum potassium sulfate in distilled water, incubated for 60 min at room temperature after pH adjustment to 6.5 using 10 N NaOH, and centrifuged at 750 × g for 5 min. The OVA/alum pellet was re-suspended to the original volume in distilled water. All mice were immunized on 2 different days (e.g., on day 0 and on day 7) by intraperitoneal injection of 0.2 mL alumprecipitated antigen containing 100μg of OVA bound to 4 mg of aluminum hydroxide in PBS. Seven days after the second challenges, intratracheal injection of 100 μL (250μg/ mL) of OVA (on day 14) were administered in the back of the tongue. Starting from the 3rd week (on day 21), OVA solution was administered into the nasal cavity and the respiratory tract using a nebulizer every 30 minutes for 1 day, 3 times per week, for 3 weeks (1% OVA in normal saline for first 2 weeks and 2% OVA in normal saline for last 1 week) (Fig. 2).
5. Classification of experimental groups
Animals were classified into 5 groups (n = 5 for each). 1. Normal group treated with PBS. 2. An OVA control group treated with OVA. 3. An OVA + Cyclosporine A (CsA) group treated with OVA and Cyclosporine A. 4. An OVA + CLEO (1%) group treated with OVA and 1% CLEO. 5. An OVA + CLEO (3%) group treated with OVA and 3% CLEO. Seven days after the sensitization by intratracheal injection with OVA, the normal group was exposed to aerosolized PBS and the OVA control group was exposed to aerosolized OVA for 30min/day, 3 days/week, for 3 weeks, in a polycarbonate chamber (40cm × 25cm × 27cm). The OVA + CLEO (1% or 3%) groups were exposed to CLEO for 30min/day, 3 days/week, for 3 weeks after OVA sensitization, in a polycarbonate chamber. Cyclosporin A (10mg/kg) was orally administered 3 times a week for the last 3 weeks as a positive control to the OVA-CsA group after OVA sensitization (Fig. 2).
6. Determination of AHR
AHR in the mice was estimated using the Buxco system. During the 3-week treatment period, CLEO was inhaled and the final aerosolized 2% OVA solution was sprayed. Then, 24 h later, MCH (bronchoconstrictor) aqueous solution (3.125, 6.25, 12.5, and 25mg/mL concentrations) was aerosolized and administered to 5 groups of mice, in which airway reactivity was observed for 15 minutes. Penh was calculated as follow :
Penh = Enhanced Pause, PEF = Peak Expiratory Flow, PIF = Peak Inspiratory Flow, Te = Expiratory Time, Rt = Time to expire 65% of volume (Lim and Kim, 2012)].
7. Collection of blood and BALF
After determination of AHR, the mice were anesthetized by 10% chloral hydrate with intraperitoneal injection, and blood was taken by the cardiac puncture method. Serum was obtained via centrifugation (3,000 rpm for 10 minutes) and stored at –70°C for use during the experiment.
BALF was obtained by injecting 10% fetal bovine serum Dulbecco’s modified Eagle’s medium (DMEM) cultured solution at 37°C into the respiratory tract and then extracting it; this process was repeated 3 times. A hemocytometer (ThermoFisher Scientific, Ashevile, NC, USA) was used to investigate the total number of cells in the BALF, which was centrifuged (400 g for 4minutes) using a Cellspin cytospin centrifuge (Hanil, Incheon, Korea). BALF cells were placed on glass slides and stained with Diff-Quik to investigate the numbers of eosinophils present among the white blood cells. BALF supernatant was stored at –70°C until it was used during the experiment.
8. Enzyme-linked immunosorbent assay (ELISA)
8. Enzyme-linked immunosorbent assay (ELISA) The levels of Th2 cytokines (IL-4, IL-5 and IL-13) in the BALF and splenocyte culture supernatant, and OVAspecific IgE level in serum were measured using ELISA kits (R&D System, Minneapolis, MN, USA). Each antibody was diluted in coating buffer; then, 100 μL was added to each microwell and kept overnight at 4°C. After washing each well 3 times with 200 μL of washing buffer (0.05% Tween 20 in PBS), 100 μL of the cell culture supernatant was dispensed. After incubation at room temperature for 1 hour and washing twice with washing buffer, solution (100 μL) of streptavidin-HRP conjugated antibody was added; then, samples were left again for 1 hour at room temperature, followed by another wash. To this, 100 μL aliquots of TMB were added and left in darkness for 30 minutes. Finally, 50 μL of stop solution was prepared and added, and the absorbance was measured by the ELISA reader at 450nm.
9. Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM). Statistical analysis of comparisons between groups was performed using the Student t-test. Significance was assessed as p < 0.05 or more (*p < 0.05, **p < 0.01).
RESULTS AND DISCUSSION
1. Analysis of CLEO constituents
The constituents of CLEO were analyzed using GC-MS and the results are shown in Table 1 and Fig 1. Terpenoid components of identified constituents made up more than 80% as a relative ratio to the peak area percentage. Among the terpenes present, the sesquiterpenes represented 39.35%, the monoterpenes represented 37.10%, and the diterpenes represented 3.67%. These results were almost the same to a previous study (Yang et al., 2002).
Table 2 indicated the results of the contents of α- pinene, limonene, bornyl acetate, and terpinene-4-ol among CLEO constituents, and method validation. The α-pinene content was 39.7 ± 1.39mg/mL, limonene content was 40.9 ± 1.36mg/mL, bornyl acetate content was 57.1 ± 1.87mg/mL, and terpinene-4-ol content was 18.4 ± 0.95mg/mL. The calibration curve showed excellent linearity, with a correlation coefficients 0.995. The accuracy showed a high recovery rate of 97.7 to 103.9%, and precision (coefficient of variation (CV)) was less than 5%.
It was reported that α-pinene, a component of CLEO, can dose-dependently suppress the nuclear translocation of the LPS-induced inflammatory signaling molecule NF-κB and partially control its expression in the human monocytic THP-1 cell line (Zhou et al., 2004), and limonene suppresses LPS-induced inflammatory mediators NO and PGE2 in RAW 264.7 cells (Yoon et al., 2009). In addition, in a study of the anti-inflammatory effect of tea tree essential oil, it was reported that terpinene-4-ol suppressed the production of LPS-induced tumor necrosis factor-α (TNF-α), IL-1β, IL-8, IL-10, and PGE2 (Hart et al., 2000). Theophyllin, a component of CLEO, have been reported to stimulate the regulation of the Na+-K+ pump current and cystic fibrosis transmembrane conductance regulator current by protein kinase A and B (Kottra & Vank, 2003), to be able to decrease AHR effectively (Aaronson et al., 1998), and to treat anaphylactic reactions induced by the liberation of different inflammatory mediators (Klimek, 2008). These components of CLEO are thought to possess the potential to alleviate airway inflammatory symptoms in asthma.
2. Effect of AHR by CLEO
AHR is a characteristic of asthma and indicates the degree of airflow limitation caused by hypersensitive reactions to stimuli that cause airway constriction (Kim, 2009). To measure AHR in this study, the Buxco system was used to administer varying concentrations of the airway constrictor methacholine (MCH) via spraying to each of the treatment groups, which was followed by measurement of the Penh value, which indicates the degree of airway constriction. A comparison of the measured values is shown in Fig. 3.
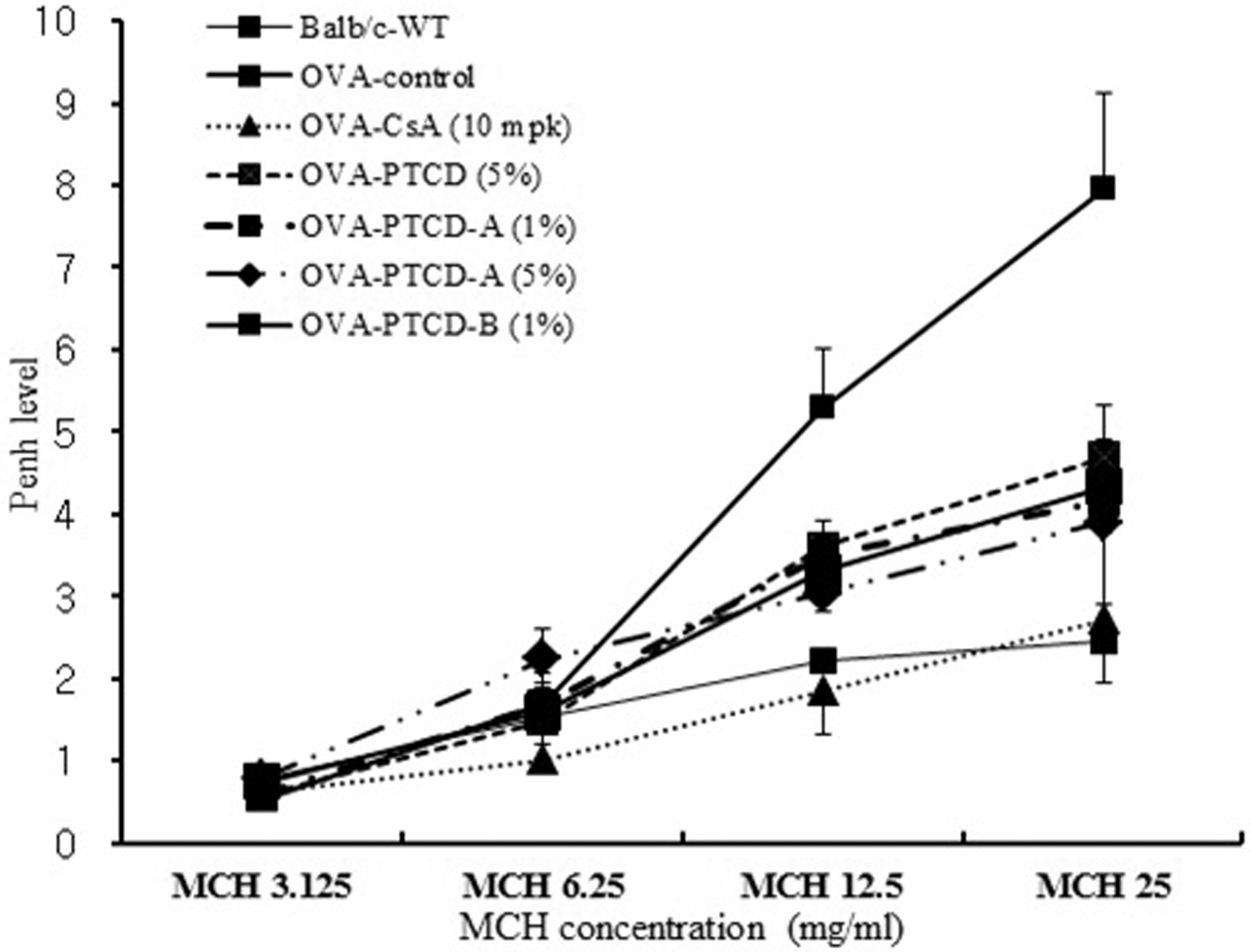
Effect of CLEO on airway hyperresponsiveness after treating methacholine.Values are expressed as mean ± SE (n = 5). Statistical analysis of data was analyzed using Student's t-test. #p < 0.05 and ##p < 0.01 compared with normal group. *p < 0.05 compared with control group. Normal; Balb/c mice, OVA-CT; OVA inhalation, OVA-CsA; OVA + cyclosporine A (10mg/kg), OVA-CLEO (3%, 1%); OVA + Chamaecyparis obusa leaf essential oil (3%, 1%), MCH; Methacholine.
The Penh value of the OVA-CT group was increased in a dose-dependent manner at MCH spray concentrations of 3.125, 6.25, 12.5, and 25.0mg/mL, and at concentrations of 3.125, 12.5, and 25mg/mL the Penh value was significantly increased in comparison to the control group (p < 0.05, p < 0.05, p < 0.01). The Penh value of the OVA-CLEO (1%) group was not significantly different from that of the OVA-CT group, but did show a decrease of approximately 11 ~ 41%. The Penh value of the OVA-CLEO (3%) group was significantly decreased by MCH spray at concentrations of 12.5 and 25mg/mL, compared to the OVA-CT group (p < 0.05). In addition, the OVA-CLEO (3%) group was compared to a group treated with the asthma medication cyclosporin A and the normal group; at all concentrations, the Penh values of the groups were similar. Therefore, according to the results of this study, 3% CLEO was determined to be effective in MCH-induced increases in AHR.
3. Effects of inflammatory cells in BALF by CLEO
In the airway of an asthma patient, the shedding of epithelial cells and infiltration of inflammatory cells (Kay, 2005; Lu et al., 2010; Possa et al., 2013), along with eosinophils with inflammatory proteins and leukotrienes, can accelerate direct damage to the airway epithelial cells, as well as cause bronchial spasms, vascular permeability, and edema. In addition, the number of eosinophils in the BALF is reported to be directly correlated with the severity of asthma (Cho et al., 1991). In this study, the total number of cells and eosinophils in mouse BALF was analyzed, and the results are shown in Table 3.
The total number of cells in the BALF of the OVA-CT group was significantly increased (p < 0.05) in comparison to the normal group, and the total number of cells in the BALF of the OVA-CsA group was significantly decreased (p < 0.05) in comparison to the OVA-CT group. The total number of cells in the BALF of the OVA-CLEO (1%) group was decreased approximately 10% in comparison to the OVA-CT group. The total number of cells in the BALF of the OVA-CLEO (3%) group was significantly decreased (p < 0.05) in comparison to the OVA-CT group. Thus, 3% CLEO effectively inhibited the proliferation of inflammatory cells that infiltrate the lung and the airway as an inflammatory response in OVA-challenged mice.
The number of eosinophils in the BALF of the OVACT group was significantly increased (p < 0.001) in comparison to the normal group, and the number of eosinophils in the BALF of the OVA-CsA group was significantly decreased (p < 0.001) in comparison to the OVA-CT group. The number of eosinophils in the BALF of the OVA-CLEO (1%) group was decreased by approximately 22% in comparison to the OVA-CT group. The number of eosinophils in the BALF of the OVACLEO (3%) group was decreased approximately 27% in comparison to the OVA-CT group. These decreases in the numbers of eosinophils in the BALF are evidence that CLEO provided some level of relief against eosinophilic airway inflammation.
4. Effect of a specific anti-OVA IgE in serum by CLEO
IgE antibody is a type I hypersensitivity reactionmediating substance that is closely tied to allergic asthma. After IgE produced in the B cells binds with high-affinity IgE receptors on the surfaces of mast cells and basophils in the peripheral blood, attachment of allergic antigens to IgE causes mast cells to release various inflammatory mediators, such as histamine, prostaglandins, leukotrienes, and cytokines. These inflammatory mediators induce mucosal edema, mucus production, and smooth muscle cell contraction in the bronchial airway (Oettgen and Geha, 2001). In this study,the levels of specific anti-OVA IgE in serum of mice were measured, and the results are shown in Table 4.
The level of specific anti-OVA IgE in serum of the OVA-CT group was significantly increased (p < 0.01) in comparison to the level of specific anti-OVA IgE in serum of the normal group. and the level of specific anti- OVA IgE in serum of the OVA-CsA group was significantly decreased (p < 0.05) in comparison to that of the OVA-CT group. The level of specific anti-OVA IgE in serum of the OVA-CLEO (1%) group was 12% less than that of the OVA-CT group, and the level of specific anti-OVA IgE in serum of the OVA-CLEO (3%) group was significantly decreased (p < 0.05) in comparison to that of the OVA-CT group.
In this study both 1% and 3% OVA-CLEO groups showed decreased level of specific anti-OVA IgE in serum, and the decrease was statistically significant for the OVA-CLEO (3%) group (p < 0.05). These results indicate that 3% CLEO has the capability to suppress airway constriction and inflammation, and although 1% CLEO did not produce a significant difference in these measurements, it is believed to be effective in alleviating airway constriction and airway inflammation by lowering the levels of specific anti-OVA IgE.
5. Effect of IL-5 and IL-13 levels in BALF by CLEO
IL-5 is responsible for differentiating immature eosinophils into mature cells and reportedly increases their intravascular release and survival (Yamaguchi et al., 1988), and IL-13 induces IgE production in B cells and is involved in eosinophilic airway inflammation (van der Pouw Kraan et al., 1998). In addition, it was reported that IL-13 plays an important role in causing airway tissue inflammation, mucus hypersecretion, bronchial fibrosis, metaplasia of goblet cells, and smooth muscle cell proliferation in mice (Zhu et al., 1999). In this study, the levels of IL-5 and IL-13 in BALF were measured, as shown in Table 5.
IL-5 in the BALF of the OVA-CT group was significantly increased (p < 0.01) in comparison to the normal group, and IL-5 in the BALF of the OVA-CsA group was significantly decreased (p < 0.05) in comparison to the OVA-CT group. IL-5 in the BALF of the OVACLEO (3%) group was significantly decreased (p < 0.05) in comparison to the OVA-CT group, and IL-5 in the BALF of the OVA-CLEO (1%) group was also significantly decreased (p < 0.05) in comparison to the OVA-CT group.
IL-13 in the BALF of the OVA-CT group was significantly increased (p < 0.01) in comparison to the normal group, and IL-13 in the BALF of the OVA-CsA group was significant decreased (p < 0.05) in comparison to the OVA-CT group. IL-13 in the BALF of the OVACLEO (3%) and OVA-CLEO (1%) groups was significantly decreased (p < 0.05) in comparison to the OVA-CT group. In this study, decreased levels of IL-5 and IL-13 in the BALF of the OVA-CLEO (1%) group and OVA-CLEO (3%) group were verified, leading to the determination that CLEO effectively suppressed the inflammatory response, alleviating eosinophilic airway inflammation and mitigating the unique histological changes associated with asthma. In conclusion, CLEO have an alleviating effect on asthma-like symptoms such as AHR and AI. Further studies about antiasthmatic effect are necessary on the focus of single component of CLEO.
ACKNOWLEDGMENTS
This work was supported by a research grant from Chungbuk National University in 2013.
REFERENCES
-
Aaronson, D, Kaiser, H, Dockhorn, R, Findlay, S, Korenblat, P, Thorsson, L, Kallen, A, Effects of budesonide by means of the turbuhaler on the hypothalmicpituitary-adrenal axis in asthmatic subjects: A dose-response study, Journal of Allergy Clinical Immunology, (1998), 101, p312-319.
[https://doi.org/10.1016/s0091-6749(98)70241-6]

-
An, BS, Kang, JH, Yang, H, Jung, EM, Kang, HS, Choi, IG, Park, MJ, Jeung, EB, Anti-inflammatory effects of essential oils from Chamaecyparis obtusa via the cyclooxygenase-2 pathway in rats, Molecular Medicine Reports, (2013), 8, p255-259.
[https://doi.org/10.3892/mmr.2013.1459]

-
Bateman, ED, Hurd, SS, Barnes, PJ, Bousquet, J, Drazen, JM, FitzGerald, M, Gibson, P, Ohta, K, O'Byrne, P, Pedersen, SE, Pizzichini, E, Sullivan, SD, Wenzel, SE, Zar, HJ, Global strategy for asthma management and prevention: GINA executive summary, European Respiratory Journal, (2008), 31, p143-178.
[https://doi.org/10.1183/09031936.00138707]

- Cho, SE, A study on the immunomodulatory effects of Chamaecyparis obtusa leaves on NC/Nga mice as models for atopic dermatitis, Journal of Korean Beauty Society, (2012), 18, p78-89.
- Cho, SH, Cho, YJ, Choi, DC, Min, KU, Kim, YY, Kim, KY, Bronchoalveolar lavage profiles and pathologic findings in bronchial asthmatics according to severity, Korean Journal of Asthma, Allergy and Clinical Immunology, (1991), 11, p466-479.
-
Hart, PH, Brand, C, Carson, CF, Riley, TV, Prager, RH, FinlayJones, JJ, Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia(tea tree oil), suppresses inflammatory mediator production by activated human monocytes, Journal of Inflammation Research, (2000), 49, p619-626.
[https://doi.org/10.1007/s000110050639]

-
Holgate, ST, Pathogenesis of asthma, Clinical & Experimental Allergy, (2008), 38, p872-897.
[https://doi.org/10.1002/9781444300918.ch78]

-
James, AL, Par, PD, Hogg, JC, The mechanics of airway narrowing in asthma, American Review of Respiratory Disease, (1989), 139, p242-246.
[https://doi.org/10.1164/ajrccm/139.1.242]

-
Kay, AB, The role of eosinophils in the pathogenesis of asthma, Trends in Molecular Medicine, (2005), 11, p148-152.
[https://doi.org/10.1016/j.molmed.2005.02.002]

- Kim, HS, Han, SK, Mang, JY, Evaluations on the deodorization effect and antibacterial activity of Chamaecyparis obtusa essential oil, Korean Journal of Odor Research and Engineering, (2009), 8, p111-117.
-
Kim, JY, Kim, SY, Kwon, HM, Kim, CH, Lee, SJ, Park, SC, Kim, KH, Comparison of antioxidant and anti-inflammatory activity on chestnut, chestnut shell and leaves of Castanea crenata extracts, Korean Journal of Medicinal Crop Science, (2014), 22, p8-16.
[https://doi.org/10.7783/kjmcs.2014.22.1.8]

-
Kim, SH, Lee, SY, Hong, CY, Gwak, KS, Yeo, HM, Lee, JJ, Choi, IG, Whitening and antioxidant activities of essential oils from Cryptomeria japonica and Chamaecyparis obtusa, Journal of The Korean Wood Science and Technology, (2011), 39, p291-302.
[https://doi.org/10.5658/wood.2011.39.4.291]

- Kim, WK, Airway hyperresponsiveness in bronchial asthma, The DongGuk Journal of Medicine, (2009), 16, p55-62.
- Klimek, L, Modern treatment concepts of anaphylactic reactions, Medizinische Monatsschrift fur Pharmazeuten, (2008), 31, p291-296.
- Kottra, G, Vank, C, The forskolin-induced opening of tight junctions in Xenopus gallbladder epithelium is mediated by protein kinase C, Cellular and Molecular Biology(Noisy-le-Grand. France), (2003), 49, p33-43.
-
Kwak, HG, Lim, HB, Ligustrum lucidum fruits extract inhibits acute pulmonary inflammation in Mice, Korean Journal of Medicinal Crop Science, (2013), 21, p323-328.
[https://doi.org/10.7783/kjmcs.2013.21.5.323]

-
Lee, SE, Lee, JH, Kim, JK, Kim, GS, Kim, YO, Soe, JS, Choi, JH, Lee, ES, Noh, HJ, Kim, SY, Anti-inflammatory activity of medicinal plant extracts, Korean Journal of Medicinal Crop Science, (2011), 19, p217-226.
[https://doi.org/10.7783/kjmcs.2011.19.4.217]

- Lee, HO, Haek, SH, Han, DM, Antimicrobial effects of Chamaecyparis obtusa essential oil, Korean Journal of Microbiology and Biotechnology, (2001), 29, p253-257.
- Lim, HB, Kim, SH, The effect of crude saponins of korean red ginseng against airway inflammation and airway hyperesponsiveness induced by diesel exhaust particles in mice, Korean Journal of Medicinal Crop Science, (2009), 17, p90-96.
-
Lim, HB, Kim, SH, Antiasthmatic effects on Scutellaria baicalensis Georgi extracts against airway inflammation and hyperresponsiveness induced by diesel exhaust particles with ovalbumin sensitization, Korean Journal of Medicinal Crop Science, (2012), 20, p129-135.
[https://doi.org/10.7783/kjmcs.2012.20.2.129]

-
Lu, Y, Sjstrand, M, Malmhll, C, Rdinger, M, Jeurink, P, Ltvall, J, Bossios, A, New production of eosinophils and the corresponding TH1/TH2 balance in the lungs after allergen exposure in BALB/c and C57BL/6 mice, Scandinavian Journal of Immunology, (2010), 71, p176-185.
[https://doi.org/10.1111/j.1365-3083.2009.02363.x]

- Miller, AL, The etiologies, pathophysiology, and alternative/complementary treatment of asthma, Alternative Medicine Review, (2001), 6, p20-47.
- Nelson, HS, Davies, DE, Wicks, J, Powell, RM, Puddicombe, SM, Holgate, ST, Airway remodeling in asthma: New insights, Journal of Allergy and Clinical Immunology, (2003), 111, p215-225.
-
Oettgen, HC, Geha, RS, IgE regulation and roles in asthma pathogenesis, Journal of Allergy and Clinical Immunology, (2001), 107, p429-441.
[https://doi.org/10.1067/mai.2001.113759]

-
Possa, SS, Leick, EA, Prado, CM, Martins, MA, Tibrio, IF, Eosinophilic inflammation in allergic asthma, Frontiers in Pharmacology, (2013), 4, -46.
[https://doi.org/10.3389/fphar.2013.00046]

-
Pyun, BY, Pharmacologic treatment of childhood asthma, Journal of the Korean Medical Association, (2007), 50, p1130-1135.
[https://doi.org/10.5124/jkma.2007.50.12.1130]

-
Salpeter, SR, Ormiston, TM, Salpeter, EE, Meta-analysis: Effect of long-acting β-agonists on severe asthma exacerbations and asthma-related deaths, Annals of Internal Medicine, (2006), 144, p904-912.
[https://doi.org/10.7326/0003-4819-144-12-200606200-00126]

-
Selli, S, Cayhan, GG, Analysis of volatile compounds of wild gilthead sea bream(Sparus aurata) by simultaneous distillation-extraction(SDE) and GC-MS, Microchemical Journal, (2009), 93, p232-235.
[https://doi.org/10.1016/j.microc.2009.07.010]

-
Spitzer, WO, Suissa, S, Ernst, P, Horwitz, RI, Habbick, B, Cockcroft, D, Boivin, JF, Mcnutt, M, Buist, S, Rebuck, AS, The use of β-agonists and the risk of death and near death from asthma, New England Journal of Medicine, (1992), 326, p501-506.
[https://doi.org/10.1056/nejm199202203260801]

-
van der Pouw Kraan, TCTM, van der Zee, JS, Boeije, LCM, de Groot, ER, Stapel, SO, Aarden, LA, The role of IL-13 in IgE synthesis by allergic asthma patients, Clinical & Experimental Immunology, (1998), 111, p129-135.
[https://doi.org/10.1046/j.1365-2249.1998.00471.x]

-
Yamaguchi, Y, Hayashi, Y, Sugama, Y, Miura, Y, Kasahara, T, Kitamura, S, Torisu, M, Mita, S, Tominaga, A, Takatsu, K, Suda, T, Highly purified murine interleukin 5(IL-5) stimulates eosinophil function and prolongs in vitro survival. IL-5 as an eosinophil chemotactic factor, Journal of Experimental Medicine, (1988), 167, p1737-1742.
[https://doi.org/10.1084/jem.167.5.1737]

- Yang, JK, Kang, BK, Kim, TH, Hong, SC, Seo, WT, Choi, MS, Efficient extraction method and analysis of essential oil from softwood leaves, Korean Society for Biotechnology and Bioengineering Journal, (2002), 17, p357-364.
-
Yoon, WJ, Lee, NH, Hyun, CG, Limonene suppresses lipopolysaccharide-induced production of nitric oxide, prostaglandin E2, and pro-inflammatory cytokines in RAW
264.7 macrophages, Journal of Oleo Science, (2009), 59, p415-421.
[https://doi.org/10.5650/jos.59.415]

- Zhou, JY, Tang, FD, Mao, FGG, Bian, RL, Effect ofpinene on nuclear translocation of NF-B in THP-1 cells, Acta Pharmacologica Sinica, (2009), 59, p415-421.
-
Zhu, Z, Homer, RJ, Wang, Z, Chen, Q, Geba, GP, Wang, J, Zhang, Y, Elias, JA, Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production, Journal of Clinical Investigation, (1999), 103, p779-788.
[https://doi.org/10.1172/jci5909]