
Effects of Enhanced Light Transmission Rate During the Early Growth Stage on Plant Growth, Photosynthetic Ability and Disease Incidence of Above Ground in Panax ginseng
© The Korean Society of Medicinal Crop Science All rights reserved
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License ( http://creativecommons.org/licenses/by-nc/3.0 ) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
This study was performed to investigate the effects of enhanced light transmission on plant growth, photosynthetic ability, and disease tolerance to leaf blight, anthracnose in ginseng (Panax ginseng C. A. Meyer, Araliacease family) during the early growth stage (April to June). The photosynthetic ratio, stomatal conductance, and stem diameter of plants grown under a shade net with 15% light transmission rate showed an increasing trend compared to the control plants (5% light transmission rate) although the growth of the aerial parts were not influenced significantly. Plant height, stem length, and leaf length of treated plants were not significantly different from those of the control plants. Root parameters, such as root length, diameter, and weight of treated plants increased significantly compared to the control. Yield performance (187.4 ㎏• 10 a−1) of treated plants was 55.5% higher than that of the control (150.4 ㎏• 10 a−1). Additionally, disease severity scores of treated plants were lower than those of the control plants, revealing higher survival rates. To retain high yield potential and enhance the level of disease tolerance in ginseng, we suggest the increase of light transmission rate during the early growth stage.
Keywords:
Light Transmission, Leaf Blight, Anthracnose, GinsengINTRODUCTION
Ginseng (Panax ginseng C. A. Meyer, Araliacease family) is one of the most important perennial herbaceous plants because of its widely known medicinal efficacies. It has been used for long in herbal medicine and therapy, especially in Asian countries such as Korea, China, and Japan. Ginseng growth and yield potential are affected by a variety of factors such as light intensity, temperature, soil moisture content, and inorganic nutrient (Cheon et al., 1991; Kim, 1991; Proctor et al., 2010; Lee et al., 2007).
Light saturation point of Korean ginseng is 11,000 lux at 15°C - 20°C and about 9,500 lux above 25°C. The light saturation point, as well as photosynthetic capacity, was shown to be significantly influenced by leaf and air temperature (Hyun et al., 1993). However, light intensity is probably the most important factor to promote photosynthetic ability in ginseng and is related with growth and yield potential (Proctor et al., 2010). When the ginseng grows under low light intensity, root growth is remarkably decreased due to slow assimilation with declining photosynthetic ability (Cheon et al., 2003; Hwang and Hyun, 1989). Ginseng is a semi-shade plant with high death rate at high light intensities and its cultivation is performed under shade conditions during the entire growth season. Cheon (1989) reported that when light intensity is too high, chlorophyll content and photosynthetic ability, which are highly related with growth and yield potential, are remarkably decreased. Ginseng leaves that are exposed to monochromatic light, such as far-red and red, for a long time show leaf-bleaching symptoms (Lee et al., 1999). Ahn et al. (1994) reported that the content of thylakoid membrane proteins in leaves exposed to high light intensity is lower than in the leaves exposed to low light intensity. Cho et al. (2008) reported that when ginseng is grown at high light intensity, leaves show symptoms of physiological disorder, such as the chlorosis; the hypodermis is remarkably increased with less phloem development inside the vascular tissue; the roots show epidermal destruction caused by decreased cambium development.
Two major diseases in ginseng, anthracnose blight disease caused by Colletotrichum gloeosporioides and leaf blight disease by Alternaria panax, decrease yield performance because of defoliation before root enlargement. Disease outbreak and growth of pathogens are highly related with light quality, air temperature, soil moisture, humidity, and plant growth stage (Oh et al., 1987). However, Cheon et al. (2003) showed that leaf blight severity and defoliation degree of plants grown under a shade net with changing light transmission rate are lower compared to those grown under a shade net with fixed light transmission in the treatment plot of fixing light transmittance during ginseng growing seasons. Therefore, the objective of this study was to examine the effect of enhanced light transmission rate during the early growth stage on plant growth, photosynthetic ability, and disease tolerance to anthracnose blight and leaf blight in ginseng.
MATERIALS AND METHODS
1. Plant growth and treatments
Two-year-old ginseng (Panax ginseng C. A. Meyer, Araliacease family) seedling roots used in this study were obtained from a specialized grower. Selected seedling roots of 10 - 12 g were transplanted into a field of clay loam soil at a density of 33 plants • m–2 (6 plants × 9 rows, plot size 1.62m2). Plants were grown under a blue polyethylene shade net with 5% light transmission rate, following Rural Development Administration (RDA, 2011) ginseng cultivation standard farming method. The blue polyethylene shading net was opened from 17:00 pm the day before to 10:00 am the next morning for enhancing light transmission inside the shading structures without removing the blue shading sheets that reflected sunlight and protected from the rain. This treatment was conducted for about 60 days from April 23 to June 23 in 2014 at the Agricultural Experimental station of the National Institute of Horticultural and Herbal Science, Soi-myeon, Emsoung, Chungbuk Province, Korea.
2. Growth parameters and photosynthetic ability of ginseng plants
Data on growth parameters of the aerial parts including in plant height, stem length, stem diameter, leaf length, and leaf width were collected on July 20. Data on root growth parameters, such as root length, diameter, weight, and the number of lateral roots were collected after harvest on October 20. All growth characteristics were examined in 10 plants and replicated 4 times per treatment plot.
Ginseng leaves were collected between 9:00 am and 10:00 am, when the photosynthetic ability is the highest, on June 20. Leaf sections (4 × 4mm) were put in 2.5% glutaraldehyde solution and stored for 90 min 4°C for the first tissue fixation. Then, they were rinsed with 0.1M phosphate buffer (pH 7.2), for 4 - 5 times at intervals of 15min. Leaf sections were put in 1% osmium tetroxide solution and kept for 90min at 4°C for the second tissue fixation. They were rinsed with 0.1M phosphate buffer (pH 7.2) for 4 - 5 times at intervals of 20min, and treated in 0.1M phosphate buffer overnight. The surface of leaf sections were dehydrated 2 times for 45 min each with 40, 60, 80, 90, 95%, and 100% ethanol solution and dried in a critical point dryer. Before scanning electron microscopic (SEM, S- 2460N, Hitachi, Tokyo, Japan) observation, the mounted samples were gold coated using Ion-Sputter (K-450, Emitech, Ashford, England). Surface pattern and stomatal aperture of ginseng leaves were observed by SEM (S- 2460 N, Hitachi, Tokyo, Japan).
Light quantity was measured about 60cm above the ground between 8:30 am and 9:00 am by a portable illuminometer (CL-200A, Konica Minolta, Tokyo, Japan) on June 20. The light transmission ratios were calculated by the following equation:
Light transmission ratio (%) = (light quantity under shading net / external light quantit ) × 100
Soil moisture and temperature were measured at a depth of 15cm between 9:00 am and 10:00 am on June 20 by an electronic soil-temperature and moisture measuring instrument (T1000H, Mirae sensor, Seoul, Korea). Chlorophyll content of ginseng leaves was analyzed using a portable chlorophyll-measuring instrument (SPAD-502Plus, Konica Minolta, Tokyo, Japan). Net photosynthetic ratio and stomatal conductance were measured on June 20 between 8:30 am and 9:30 am by an LI-6000 portable photosynthesis system (Li-Cor, Lincoln, NE, USA) under natural light with an air influx rate of 500mol• s–1, a CO2 concentration of 400mol• s–1, a relative humidity of 40%, and a temperature of 20°C.
3. Identification and severity scoring of disease and physiological disorder
For identifying ginseng pathogens, the infected plants were put on water agar medium. Conidia and conidiophores were identified using stereoscopic and optical microscopes. Severity of leaf blight disease was scored on a 1 - 5 scale based on the proportion of leaf spot disease lesions (1; no disease symptoms observed, 2; only pencilpointed or arrested spots on leaf, 3; symptoms on 25% of leaf area, 4; symptoms on 50% of leaf area, and 5; symptoms on more than 50% of leaf area). Severity of anthracnose blight disease was also scored on a 1 - 5 scale (1; no symptom observed, 2; more than 5 spots with lesions less than 2mm in diameter, 3; less than 10 spots with lesions less than 4mm in diameter, 4; less than 10 spots with lesions less than 6mm in diameter, and 5; more than 10 spots with lesions larger than 6mm in diameter).
Severity of rusty root was scored on a 1 - 5 scale based on the proportion of reddish brown to black root lesions (1; 0%, 2; less than 10%, 3; 11 - 29%, 4; 30 - 49% and 5; more than 50%). The percentage of inside cracking and rough skin were calculated as the number of inside cracking and rough skin to the total number of plants, respectively.
4. Statistical analysis
The experiment was analyzed as a randomized block design using SAS (SAS institute Inc., Cary, NC, USA). Analysis of variance in conjunction with student’s t-test was applied to test the significance of mean differences among treatments at p < 0.05 and p < 0.01.
RESULTS AND DISCUSSION
1. Stomatal aperture and photosynthetic ability
Stomatal aperture of ginseng (Panax ginseng C. A. Meyer, Araliacease family) leaves from plants grown under a shade net with 15% light transmission rate is shown Fig. 1. Enhanced light transmission rate stimulated stomatal opening, results that were in agreement with previously reported studies (Casson and Gray 2008; Roelfsema et al., 2006). Ginseng, as an understory herb plant, has a lower compensation point and appears to be very susceptible to high light intensity and high temperature compared to other full-sun plants (Hwang and Hyun, 1989). Stomatal movement and development is not only influenced by light conditions, but also by water status, CO2 concentration, and abscisic acid (Casson and Gray, 2008; Roelfsema et al., 2006; Royer, 2001; Xu and Zhou, 2008).
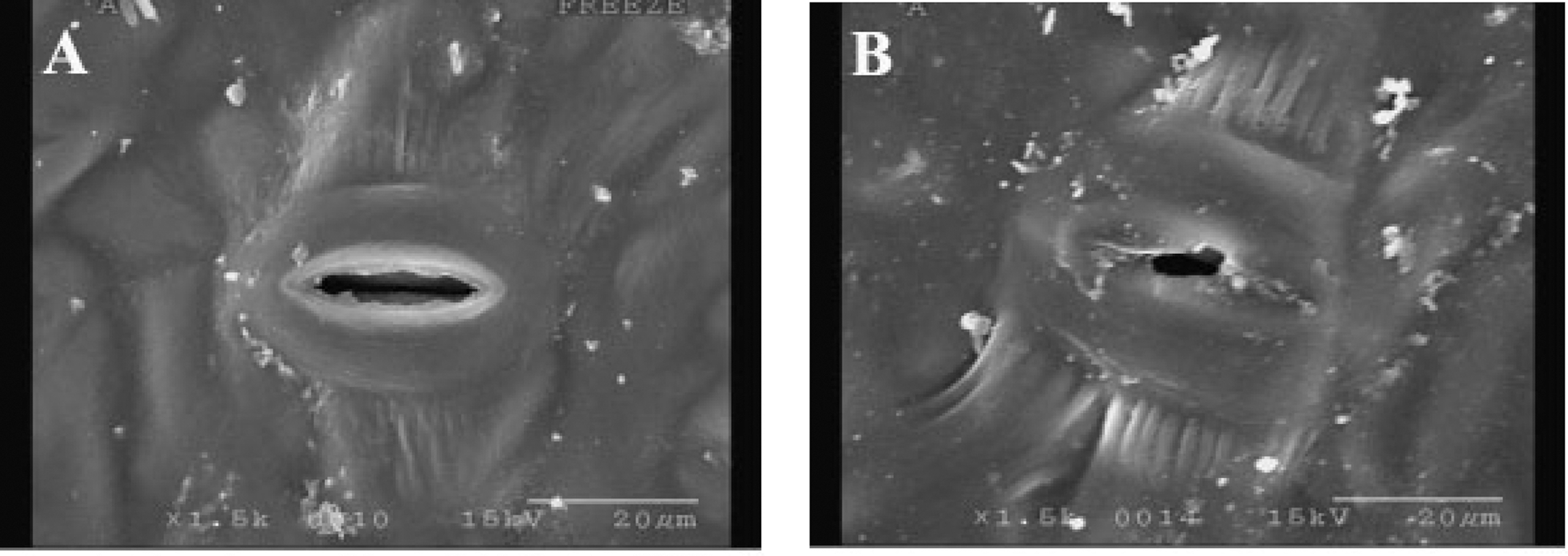
Stomatal aperture of ginseng leaves.A; stomatal aperture of ginseng leaf grown under a shade net with 15% light transmission rate, B; stomatal aperture of ginseng leaf grown under a shade net with 5% light transmission rate (control). Ginseng leaves were collected between 8:30 am and 9:00 am on June 20.
In our study, stomatal aperture was increased with increasing light intensity and temperature. Stomatal frequency (number of stomata • mm–2) and the number of guard cells are considerably affected by light intensity (Casson and Gray, 2008). In particular, those are increased with increasing light transmission and reach a peak at 15% light transmission ratio (Park, 1980). Guard cells in albino leaf patches do not respond to photosynthetic active radiation (PAR) but are sensitive to blue light, CO2, and abscisic acid (Roelfsema et al., 2006). Stomatal opening can be stimulated more by blue light than by PAR, which is associated with the photosynthetic ability of mesophyll cells and involved to stomatal opening (Assmann and Shimazaki, 1999; Roelfsema and Hedrich, 2005).
Changes in photosynthetic ability of plants grown under a shade net with 15% light transmission rate and the control plants are presented in Table 1. On June 20, light transmission rates of treatment plot and control plot were 15% and 5%, respectively, a difference that was highly significant. Jo et al. (1986) reported that ginseng plants grown under 10 - 15% light transmission ratios showed the highest photosynthetic ability.

Effect of light transmission rate (LTR) on photosynthetic ability and stomatal conductance in ginseng.
In this study, soil moisture content and temperature of the treatment plot were lower compared to the control plot. Reduction of soil moisture content and increase of soil temperature in the treatment plot was probably due to the enhanced light transmission rate that accelerated water evaporation and increased soil temperature, result that were in agreement with those reported by Cheon et al. (1991).
Net photosynthetic ratio (3.8 μmol• m–2s–1) of treated plants was higher than that of the control plants (1.1 μmol• m–2s–1), indicating a high value (0.07 μmol• m–2s–1) of stomatal conductance compared to the control (0.05 μmol• m–2s–1). The effect of environmental factors, such as light intensity, soil moisture content, and temperature of soil and air on photosynthesis in ginseng well studied. In particular, temperature and light intensity have been shown to be highly associated with increased photosynthetic ability (Kim, 1991). Lee et al. (1987) and Lee (2012) reported that most of the cultivars of Korean ginseng, including ‘Chunpoong’, ‘Yunpoong’, ‘Gopoong’, and ‘Jakyungjong’ reach the highest photosynthetic ration 15,000 lux and 20°C. Leaf temperature is also major activator of photosynthetic capacity and its optimal level is 18°C (Hwang and Hyun, 1989; Hyun et al., 1993). However, temperature and light intensity do not act separately, but interact to enhance the photosynthetic ratio in ginseng. Lee (2007) reported that the net photosynthetic ratio was largely influenced by photosynthetically action radiation (PAR) and transpiration rate was more increased in the afternoon than in the morning.
2. Growth parameters and yield performance
Ginseng plant grown under a shade net with 15% light transmission rate showed a good diversity of certain growth parameters, including stem diameter, yield, fresh root weight, root diameter, and root length compared to the control (Table 2 and 3). In previous studies, enhanced light transmission rate decreased stem length and leaf length, increased stem diameter, and decreased chlorophyll content (Cheon et al., 1991, 2003; Cho et al., 2008; Kim et al., 2012). Cheon et al. (2003) reported that leaf weight was increased with increasing light transmission rate but in general, the growth of the aerial parts shows a decreasing tendency. By contrast, Proctor et al. (2010) reported that the growth of the aerial parts continues to increase up to 30% light transmission rate. Kim et al. (1982) reported that the leaves of ginseng grown in the front row were lager than the ones in the back row because of a large amount of light. In our study, the growth parameters of the aerial parts, such as stem diameter and chlorophyll content, were significantly higher in the treatment plot than in the control plot. Stem height and leaf length decreased with increasing light intensity, while plant height and leaf length and width had no significant differences between treated plants and the control, results that were not in agreement with previous studies (Kim et al., 2012). That was probably because the unique growth parameters of ginseng develop very slowly (Jin et al., 2009); therefore, further study may be necessary to accurately investigate the same parameters at aged roots.
As the light transmission ratio increases, leaf scorches and chlorosis are rapidly accelerated in ginseng leaves, leading to early defoliation before root enlargement (Cho et al., 2008). This may occur because as the leaves are consistently exposed to high light intensity, the cuticle and the wax layers, which protect the leaf tissues from ultraviolet rays and prevent moisture loss, respectively, collapse as a result of high temperature injury, due to intense evaporation on the leaf surfaces (Lee et al., 2011; Samdur et al., 2003). Chlorophyll decomposition in ginseng leaves is caused by singlet oxygen, which is produced during photosynthesis and increasingly progresses with excessive light intensity (Yang et al., 1991).
In this study, the growth of underground parts was remarkably better in the treatment plot than control plot reported by Cheon et al. (1991, 2003), Lee et al. (1982). They showed that the development of ginseng roots increases with increasing light intensity and suggested that the optimum light transmission rate for root growth is between 10% and 15% of full sunlight. In this study, root parameters, such as root weight, root diameter, and root length of treated plants were significantly increased compared to the control plants. Yield performance (187.4kg• 10 a–1) of treated plants was 55.5% higher than that of the control plants (150.4kg• 10 a–1). The results showed that the enhanced light transmission rate appositively affected the photosynthetic ability also reported by Cheon et al. (1991) and Kim et al. (2012). However, the number of lateral roots was not significantly different between the treatment and the control plot. Our results suggested that proper management of light transmission rate in the morning hours might increase yield performance of ginseng plants.
3. Disease severity and physiological disorder
Ginseng is highly susceptible to plant diseases compared to other crops. Two major diseases in ginseng, anthracnose blight disease, caused by C. gloeosporioides, and leaf blight disease, caused by A. panax, decrease yield performance by defoliation before root enlargement (Choi et al., 2011; Han et al., 2004). These two highly destructive pathogens are air-borne and greatly stimulated by warm rainy weather conditions year round (Kang et al., 2007; Kim et al., 2008). Therefore, growers must apply fungicides, such as azoxystrobin and pyraclostrobin many times during growing season to protect the plants against defoliation and subsequent death.
The effect of enhanced light transmission rate on diseases severity is presented in Table 4 and on plant survival rate is shown in Fig. 2. Anthracnose blight and leaf blight severity on leaves and stems was associated with light intensity and leaking rate. Infection rate s also increased with increasing light intensity under warm rainy weather conditions (Cheon et al., 1991), because pathogens easily enter into the plants through the opened stomata (Chung and Bae, 1979). However, our results were not in agreement with previous studies, except for the incidence rate of leaf blight. Cheon et al. (2003) reported that ginseng showed high survival rate and tolerance to plant diseases when light intensity was enhanced during the early growth stage (April to June) and the late growth stage (September to October), but not during the summer (July to August). Cho et al. (2008) observed that cuticle layers on leaves surface, which have a protective function against pathogen infections and insect damages, are developed at higher light intensity and then the stem tissue is hardened by the increase in hypodermic cells formed inside the cortex. Ginsenoside, which is the most important secondary metabolites in ginseng plant, has been reported to be largely involved in plant defense against pathogen’s attack and in enhancement of plant growth (Nicol et al., 2002; Zhang et al., 2011). Their contents increase with an increase of light intensity (Kim et al., 2012). Thus, plant survival rate was higher in the treatment plot than the control plot, indicating a low infection rate.
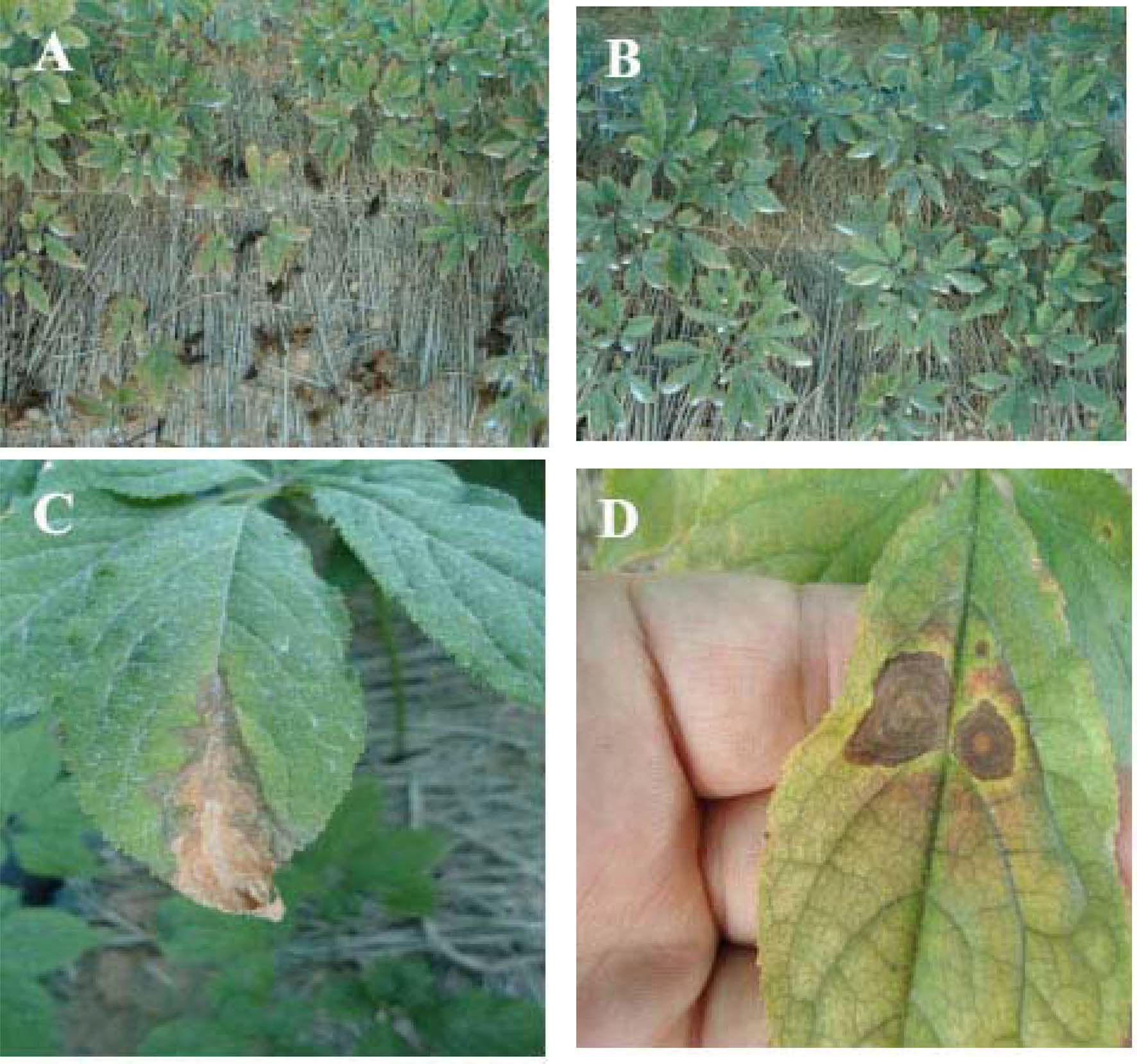
Severity of leaf blight caused by Alternaria panax and anthracnose caused by Colletotrichum gloeosporioides in ginseng.A; ginseng plants grown under a shade net with 5% light transmission rate (control), B; ginseng plants grown under a shade net with 15% light transmission rate, C; representative symptoms on caused by Alternaria panax, D; representative symptom on ginseng leaves caused by Colletotrichum gloeosporioides.

Effect of light transmission rate (LTR) on anthracnose blight and leaf blight severity and plant survival rate in ginseng.
In our study, leaf blight severity was slightly higher in the treatment plot compared to control plot, but the difference was not statistically significant. This was probably because weather conditions were unfavorable for pathogen development (Kim et al., 1990). The disease causes blighting of stem and leaf from mid-May to the end of June (Oh et al., 1987) but during these periods, amount of rainfall was very low. Becktell et al. (2005) reported that temperature and leaf wetness are requirements for disease development. Outbreaks of disease are greatly affected by a relative humidity, temperature and wetness periods (Arauz and Sutton, 1989; Fitt et al., 1989). Therefore, disease development was insufficient for evaluating plant tolerance against leaf blight in this study.
The effect of enhanced light transmission on physiological disorders in ginseng is presented in Table 5. The severity of physiological disorders, such as inside cracking root and rough skin, resulting in low quality red ginseng, significantly differed between the treatments plot and the control plot. The severity of rusty root, resulting in low quality, root rot, and decreased yield performance was not significantly different between the treatment plot and the control plot, results that were not in agreement with previous studies (Kim et al., 1990). The severity of physiological disorders, including rusty root and cracking root, was higher in the treatment plot than that in the control plot. Otherwise, rough skin and root rot caused by Pythium ultimum were lower in the treatment plot than in the control plot. Kim et al. (1990) mentioned that physiological disorders, such as inside root cavity and inside root cracking were increased with increasing light intensity. The differences between anabolism and catabolism are because high light intensity accelerates respiration. Thus, the accumulation of starch granules inside the root was inhibited by the significant difference between the two metabolic processes during root enlargement stage. Cho et al. (2008) observed that an increase in the light transmission rate over 15% leads to the destruction of root epidermis and the inhibition of cambium development and phloem development inside the vascular cylinder, which is the channel of mass transportation in plants.
ACKNOWLEDGEMENTS
This work was carried out with the support of Cooperative Research Program for Agriculture Science and Technology Development(PJ00946802) Rural Development Administration, Republic of Korea.
REFERENCES
- Ahn, JS, Cho, BG, Park, H, Kim, WK, Changes in chloroplast ultrastructure and thylakoid membrane proteins by high light in ginseng leaves, Journal of Plant Biology, (1994), 37, p285-292.
-
Arauz, LF, Sutton, TB, Influence of temperature and moisture on germination of ascospores and conidia of Botryoshaeria obtusa, Phytopathology, (1989), 79, p667-674.
[https://doi.org/10.1094/phyto-79-667]

-
Assmann, SM, Shimazaki, KI, The multisensory guard cell. Stomatal responses to blue light and abscisic acid, Plant Physiology, (1999), 119, p809-816.
[https://doi.org/10.1104/pp.119.3.809]

-
Becktell, MC, Daughtrey, ML, Fry, WE, Temperature and leaf wetness requirements for pathogen establishment, incubation period, and sporulation of Phytophthora infestans on Petunia × hybrida, Plant Disease, (2005), 89, p975-979.
[https://doi.org/10.1094/pd-89-0975]

-
Casson, S, Gray, JE, Influence of environmental factors on stomatal development, New Phytologist, (2008), 178, p9-23.
[https://doi.org/10.1111/j.1469-8137.2007.02351.x]

- Cheon, SK, Effects of light intensity and quality on the growth and quality of Korean ginseng. Ph. D. Thesis, (1989), Kyungbook National Univesity, p1-41.
- Cheon, SK, Lee, TS, Yoon, JH, Lee, SS, Effect of light transmittance control on the growth status of aerial parts during the growing season of Panax ginseng, Journal of Ginseng Research, (2003), 27, p202-206.
- Cheon, SK, Mok, SK, Lee, SS, Shin, DY, Effects of light intensity and quality on the growth and quality of Korean ginseng(Panax ginseng C. A. Meyer). I. Effects of light intensity on the growth and yield of ginseng plants, Korean Journal of Ginseng Science, (1991), 15, p21-30.
- Cho, JW, Park, HW, Kim, MJ, Kim, HH, Choi, JE, Photosynthetic, morphological and growing characteristics by shading materials in Panax ginseng C. A. Meyer, Korean Journal of Crop Science, (2008), 53, p256-260.
-
Choi, KJ, Kim, WG, Kim, HG, Choi, HW, Lee, YK, Lee, BD, Lee, SY, Hong, SK, Morphology, molecular phylogeny and pathogenicity of Colletotricum panacicola causing anthracnose of Korean ginseng, Plant Pathology, (2011), 27, p1-7.
[https://doi.org/10.5423/ppj.2011.27.1.001]

- Chung, HS, Bae, HW, Ginseng anthracnose in Korea. Factors affecting primary inoculum, growth of the pathogen, disease development and control, Korean Journal of Plant Protect, (1979), 18, p35-41.
-
Fitt, BDL, McCartney, HA, Walklate, PJ, The role of rain in dispersal of pathogen inoculum, Annual Review of Phytopathology, (1989), 27, p241-270.
[https://doi.org/10.1146/annurev.phyto.27.1.241]

-
Han, KD, Alam, S, Lee, TS, Lee, MW, Ginseng anthracnose caused by Colletotrichum dematium, Plant Pathology, (2004), 20, p196-199.
[https://doi.org/10.5423/ppj.2004.20.3.196]

- Hyun, DY, Hwang, JK, Choi, SY, Jo, JS, Photosynthetic characteristics of Panax ginseng C. A. Meyer. I. Photosynthetic response to changes of light intensity and leaf temperature, Korean Journal of Ginseng Science, (1993), 17, p240-245.
- Hwang, JK, Hyun, DY, Differences of photosynthetic ability of tobacco and ginseng leaves in accordance with light intensity, Korean Journal of Crop Science, (1989), 34, p211-219.
- Jin, HO, Kim, UJ, Yang, DC, Effect of nutritional environment in ginseng field, Journal of Ginseng Research, (2009), 33, p234-239.
- Jo, JS, Won, JY, Mok, SK, Studies on the photosynthesis of Korean ginseng. III. Effects of the light transparent rate of shading on the photosynthesis ability of Korean ginseng plant(Panax ginseng C. A. Meyer), Korean Journal of Crop Science, (1986), 31, p408-415.
- Kang, HS, Park, DS, Hwang, YK, Kim, SM, Survey on pesticide use by ginseng growers at Gangwon farmland in Korea, Korean Journal of Pesticide Science, (2007), 11, p210-215.
-
Kim, GS, Lee, SE, Noh, HJ, Kwon, H, Lee, SW, Kim, SY, Kim, YB, Effects of natural bioactive products on the growth and ginsenoside contents of Panax ginseng cultured in an aeroponic system, Journal of Ginseng Research, (2012), 36, p430-441.
[https://doi.org/10.5142/jgr.2012.36.4.430]

- Kim, HJ, Cheong, SS, Kim, DW, Park, JS, Rhy, J, Bae, YK, Yoo, SJ, Investigation into disease and pest incidence of Panax ginseng in Jeonbuk province, Korean Journal of Medicinal Crop Science, (2008), 16, p33-38.
- Kim, JM, Effects of leaf water condition on photosynthesis and respiration in ginseng plant, Journal of Basic Science Research Institute. Hyosung Women's University, (1991), 5, p95-99.
- Kim, JM, Lee, SS, Cheon, SR, Cheon, SK, Relationship between environmental conditions and the growth of ginseng plant in field. I. Productive structures as affected by planting positions and ages, Korean Journal of Crop Science, (1982), 27, p94-98.
- Kim, YH, Yu, YH, Lee, JH, Park, CS, Ohh, SH, Effect of shading on the quality of raw, red and white ginseng and the contents of some minerals in ginseng roots, Korean Journal of Ginseng Science, (1990), 14, p36-43.
- Lee, CH, Lee, JC, Cheon, SK, Kim, YT, Ahn, SB, Studies on the optimum light intensity for growth of Panax ginseng. I. Effects of light intensity on growth of shoots and roots of ginseng plants, Korean Journal of Ginseng Science, (1982), 6, p38-45.
- Lee, CY, Characteristics of photosynthesis with growing stages by different shading materials in Panax ginseng C. A. Meyer, Korean Journal of Medicinal Crop Science, (2007), 15, p276-284.
-
Lee, JS, Lee, KH, Lee, SS, Kim, ES, Ahn, IO, In, JG, Morphological characteristics of ginseng leaves in high temperature injury resistant and susceptible lines of Panax ginseng Meyer, Journal of Ginseng Research, (2011), 35, p449-456.
[https://doi.org/10.5142/jgr.2011.35.4.449]

- Lee, SS, Characteristics of photosynthesis among new cultivars of ginseng(Panax ginseng C. A. Meyer), Journal of Ginseng Research, (2012), 26, p85-88.
- Lee, SS, Cheon, SR, Lee, CH, Comparison of photosynthetic rates of Panax species and cultivars, Korean Journal of Crop Science, (1987), 32, p157-162.
- Lee, SS, Proctor, JTA, Choi, KT, Influence of monochromatic light on photosynthesis and leaf bleaching in Panax species, Korean Journal of Ginseng Science, (1999), 23, p1-7.
- Lee, SW, Hyun, DY, Park, CG, Kim, TS, Yeon, BY, Kim, CG, Cha, SW, Effect of soil moisture content on photosynthesis and yield of Panax ginseng C. A. Meyer seedling, Korean Journal of Medicinal Crop Science, (2007), 15, p367-370.
-
Nicol, RW, Traquair, JA, Bernards, MA, Ginsenosides as host resistance factors in American ginseng (Panax quinquefolius), Canadaian Journal of Botany, (2002), 80, p557-562.
[https://doi.org/10.1139/b02-034]

- Oh, SH, Yu, YH, Kim, KH, Lee, JH, Study on control of disease and insect pest on ginseng(Panax ginseng) in Korea, (1987), Korea Tobacco and Ginseng Institute, Daejeon. Korea, p144-294.
- Park, H, Physiological response of Panax ginseng to light, Proceeding of the 3rd International Ginseng Symposium, (1980), p151-170.
-
Proctor, JTA, Palmer, JW, Follett, JM, Growth, dry matter partitioning and photosynthesis in North American ginseng seedlings, Journal of Ginseng Research, (2010), 34, p175-182.
[https://doi.org/10.5142/jgr.2010.34.3.175]

-
Roelfsema, MRG, Hedrich, R, In the light of stomtal opening: New insights into ‘the Watergate’, New Phytologist, (2005), 167, p665-691.
[https://doi.org/10.1111/j.1469-8137.2005.01460.x]

- Roelfsema, MRG, Konrad, KR, Marten, H, Psaras, GK, Hartung, W, Hedrich, R, Guard cells in albino leaf patches do not respond to photosynthetically active radiation but are sensitive to blue light, CO2and abscises acid, Plant Cell and Environment, (2006), 29, p1595-1605.
-
Royer, DL, Stomatal density and stomatal index as indicators of paleoatmospheric CO2 concentration, Review of Palaeobotany and Palynology, (2001), 114, p1-28.
[https://doi.org/10.1016/s0034-6667(00)00074-9]

- Rural Development Administration(RDA), Ginseng cultivation standard farming text book-103, (2011), Rural Development Administration, Suwon. Korea, p108-149.
-
Samdur, MY, Manivel, P, Jain, VK, Chikani, BM, Gor, HK, Desai, S, Misra, JB, Genotypic differences and water deficit induced enhancement in epicuticular wax load in peanut, Crop Science, (2003), 43, p1294-1299.
[https://doi.org/10.2135/cropsci2003.1294]

-
Xu, Z, Zhou, G, Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass, Journal of Experimental Botany, (2008), 15, p3317-3325.
[https://doi.org/10.1093/jxb/ern185]

- Yang, DC, Lee, SJ, Yun, KY, Kang, YH, Activities of anti-oxidative enzymes in photobleaching of leaves from Panax ginseng C. A. Meyer, Korean Journal of Ginseng Science, (1991), 15, p139-143.
- Zhang, AH, Lei, FJ, Fang, SW, Jia, MH, Zhang, LX, Effects of ginsenosides on the growth and activity of antioxidant enzymes in American ginseng seedlings, Journal of Medicinal Plants Research, (2011), 5, p3217-3223.