
Prediction of Optimal Microwave Extraction Conditions for Functional Compounds from Agrimonia pilosa Ledeb Using Response Surface Methodology
© The Korean Society of Medicinal Crop Science. All rights reserved.
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License ( http://creativecommons.org/licenses/by-nc/3.0 ) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
In this study, microwave extraction was used, which is an effective method to extract useful bioactive substances as it requires low quantities of solvent and short time periods. The aim of this study was to determine the optimal extraction conditions for Agrimonia pilosa Ledeb.
The independent variables were ethanol concentration, microwave power, and extraction time, each of which had five levels. The dependent variables were total polyphenol and total flavonoid content, and DPPH radical scavenging activity. To determine the optimal extraction conditions for bioactive compounds, a response surface methodology was employed. Contour maps were generated from polynomial equations. The optimal conditions were then assumed by superimposing these contour maps. Based on the resulting graph, the optimal microwave extraction conditions for Agrimonia pilosa Ledeb were determined as 42 - 48% ethanol concentration, 240 - 280W microwave power, and 13 - 20 min of extraction time.
Ethanol concentration had a significant effect on microwave extraction, in terms of total polyphenol and total flavonoid content, as well as DPPH radical scavenging activity. Microwave power and extraction time influenced the total polyphenol content, but not the total flavonoid content or the DPPH radical scavenging activity.
Keywords:
Agrimonia pilosa Ledeb, Extraction, Microwave, Optimization, Response Surface MethodologyINTRODUCTION
Agrimonia pilosa Ledeb belongs to the Rosaceae family and has been used in traditional oriental medicine for tumors, hematuresis, and uterine bleeding (Bae and Sohn, 2005). Previous studies have shown that A. pilosa contains antibacterial (Bae and Sohn, 2005), anti-aging (Yoon et al., 2012), anti-oxidative, antitumor (Min et al., 2008), whitening (Kim et al., 2011a, b), and inhibitory effect on allergic reaction (Kim, 2010). It has also been reported from previous studies that A. pilosa contains compounds of ellagic acid, rutin, taxifolin, coumarin and luteolin (Pei et al., 1990; Gao et al., 2015).
Although A. pilosa was studied about medical actions and biological activity effects, there are less research about extraction condition. There are various extraction methods available, including ultrasonic, distillation, supercritical fluid and microwave extraction. Among them, microwave-assisted extraction (MAE) is considered an effective extraction technique, and it is an economical method requiring low solvent consumption and less time (Giese, 1992; Schiffmann, 1992; Kwon et al., 2000, 2007). To find optimal condition, response surface methodology (RSM), which is one of the statistical method to optimizing conditions was applied. Because RSM have an advantage when multiple variables and their interactions are evaluated (Théodore and Panda, 1995). Many researchers also used RSM for optimize process. (Lee et al., 2006, 2012).
The aim of this study is to determine the optimum extraction condition of DPPH radical scavenging activity (DPPH) and functional ingredients, including total polyphenol content (TPC) and total flavonoid content (TFC) by using microwave-assisted extraction and response surface methodology. Those results will be fundamental data to set microwave extraction condition of A. pilosa.
MATERIALS AND METHODS
1. Sample preparation
The Agrimonia pilosa Ledeb used in this study was harvested from the Gyeongsang province in Korea. The dried sample was purchased in the market. It was ground to powder by passing them through 40 and 60 mesh sieve. The ground sample was packed in sealed plastic bags and stored in a −20℃ freezer for further use.
2. Microwave-assisted extraction (MAE)
A microdigest machine (Soxwave 100, Prolabo, Fontenay, France) was used to extract total polyphenol, total flavonoid, and antioxidants. The device operates at a frequency of 2,450 MHz and is equipped with a reflux condenser. A total of 50㎖ of ethanol was mixed with 1 g of sample powder and used to fill an extraction vessel. The extraction was performed under different conditions with the following: An ethanol concentration of 0 - 100%, an MAE power of 60 - 300W, and an extraction time of 4 - 20 min. The extract was filtered through No. 1 filter paper (Whatman Co., Maidstone, England) under a vacuum and then volumed up with the solvent to 100㎖ in a 100㎖ mass flask.
3. Determination of total phenolic content (TPC)
The total phenolic content was measured by the Folin- Denis method described by Singleton et al. (1999) with some modifications. A. pilosa extract was diluted five times with distilled water. A total of 100㎕ of diluted sample was mixed with 50㎕ of Folin-Ciocalteu's reagent and 300㎕ of 2% Na2CO3. After reacting for 15 min at room temperature, 1㎖ of distilled water was added and the absorbance was measured by spectrophotometry (UV- 2550, Shimadzu Corp., Kyoto, Japan) at 725㎚. Gallic acid (Sigma-Aldrich, St. Louis, MO, USA) was used as standard (0.075 - 0.2㎍/㎖). The results were presented as ㎎ of gallic acid equivalent per g (㎎• GAE/g).
4. Determination of total flavonoid content (TFC)
The total flavonoid content was analyzed by using aluminum colorimetric assay (Teng et al., 2009). Approximately 70㎕ of extract, which was diluted two times with distilled water, was mixed with 430㎕ of distilled water, 50㎕ of 5% NaNO2 and 50㎕ of 10% Al(NO3)39H2O. After keeping it at room temperature for 6 min, 500㎕ of 1 N NaOH was added and the absorbance was measured by spectrophotometry at 510㎚. Quantitative analysis was practiced on the basis of the standard curve of rutin (Sigma-Aldrich Co., St. Louis, MO, USA). The results were expressed as ㎎ of rutin equivalent per g (㎎• RE/g).
5. Determination of DPPH radical scavenging activity (DPPH)
DPPH radical scavenging activity was assayed by using a modified version of the DPPH method by Blois (1958). To make DPPH solution, 0.0039 g of 2, 2-diphenyl-1- picrylhydrazyl (Sigma-Aldrich Co., St. Louis, MO, USA) was mixed with 100㎖ of ethanol and then It was equilibrium for 2 hr using magnetic stirrer (PC-420D, Corning Co., Corning, NY, USA). A. pilosa extract was diluted 60 times with ethanol. Approximately 100㎕ of diluted sample was mixed with 900㎕ of DPPH solution. The sample was placed in the dark at room temperature for 30min. The absorbance was measured by spectrophotometry at 517㎚. DPPH was expressed by the following equation.
6. Experimental design
Response surface methodology (RSM) was applied to optimize the extraction condition using a statistical analysis system (SAS, Version 9.2, SAS Institute Inc., Cary, NC, USA) program. The independent variables were ethanol concentration (X1), microwave power (X2), and extraction time (X3). Through single-factor experiments and central composite design (CCD), ethanol concentration (0, 25, 50, 75 and 100%), microwave power (60, 120, 180, 240 and 300W), and extraction time (4, 8, 12, 16 and 20 min) were selected as suitable conditions for extraction and were encoded under five levels (−2, −1, 0, +1 and +2) (Table 1). The CCD matrices was composed of 2 central points, 8 factorial points, and 6 axis points at a distance of ± 2 from the center, resulting in 16 set of experimental runs. The dependent variables were total polyphenol content (Y1), total flavonoid content (Y2) and DPPH radical scavenging activity (Y3).
7. Statistical analysis
The analysis was performed in triplicates. The average values were used to regression analysis by using SAS software (SAS, Version 9.2, SAS Institute Inc., Cary, NC, USA). The following equation was regression model about independent and dependent variables:
Y is predicted responses for TPC, TFC and DPPH. Xi are the independent variables and βi are regression coefficients.
For Duncan’s Multiple Range Test, IBM SPSS statistics 22 (SPSS Inc., Chicago, IL, USA) was used. It was performed at 5% probability level.
The experimental data were expressed to statistical three dimension surface and contour plots. The contour maps were overlapped to indicate optimal conditions.
RESULTS AND DISCUSSION
1. Solvent selection for microwave extraction
Solvent is one of crucial parameters during microwave extraction process. Because solvent have a influence on affinity of target ingredients and its absorption of microwave (Zhang et al., 2011).
To select proper solvent for extraction, four different solvents including distilled water, ethanol, acetone and ethyl acetate were used.
Among them, ethanol and distilled water extracts show higher contents of functional compounds and DPPH radical scavenging activity than acetone and ethyl acetate. Especially, 50% ethanol presented significantly higher values than others (Fig. 1). It was predicted that aqueous ethanol will be suitable solvent for A. pilosa extraction. For that reason, ethanol was selected as extraction solvent.
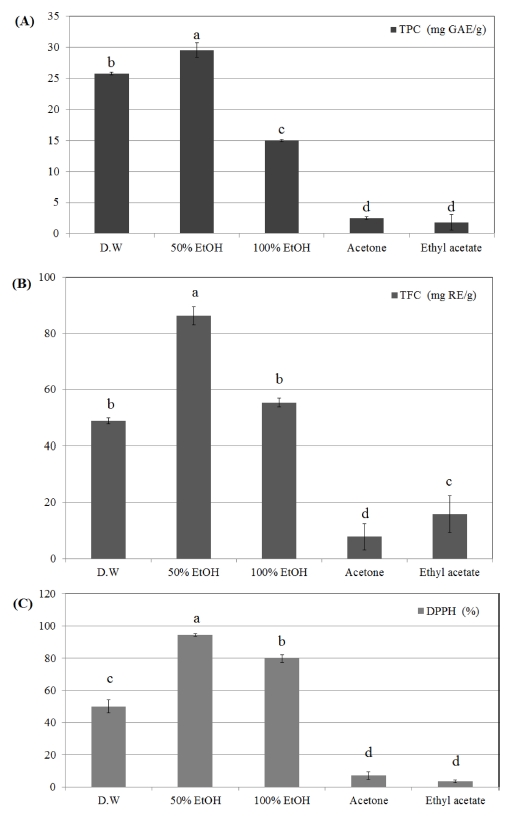
The effect of different solvents on total polyphenol content (A), total flavonoid content (B) and DPPH radical scavenging activity (C) of Agrimonia pilosa Ledeb under 5min extraction time and 180 W microwave power conditions.Mean values ± SD from triplicate separated experiments are shown. Mean with the difference letter (a - d) are by DMRT (p < 0.05).
2. Effects of extraction condition on total polyphenol content (TPC)
The experimental data for TPC are expressed in Table 2. Fitted model for TPC is listed as below:

Central composite design and experimental data of Agrimonia pilosa Ledeb during microwave extraction.
The coefficient of determination for the polynomial equation was 0.9529 and significance was less than 0.005%. The predicted maximum TPC was 37.19㎎• GAE/g under an ethanol concentration of 42.48%, microwave power of 239.04W, and extraction time of 13.25 min (Table 3). The significant effect was presented with ethanol concentration, microwave power and extraction time (Table 4). As microwave power increased, extraction of TPC was enhanced between 25 and 60% of ethanol concentration (Fig. 2). Especially, TPC showed high amount in the range of 25 - 60% of ethanol concentration. Previous study mentioned that it was important to balance the dielectric constant between solvent and solute in microwave extraction (Pare et al., 1994).

Predicted level of optimum conditions for extraction of Agrimonia pilosa Ledeb by the ridge analysis.
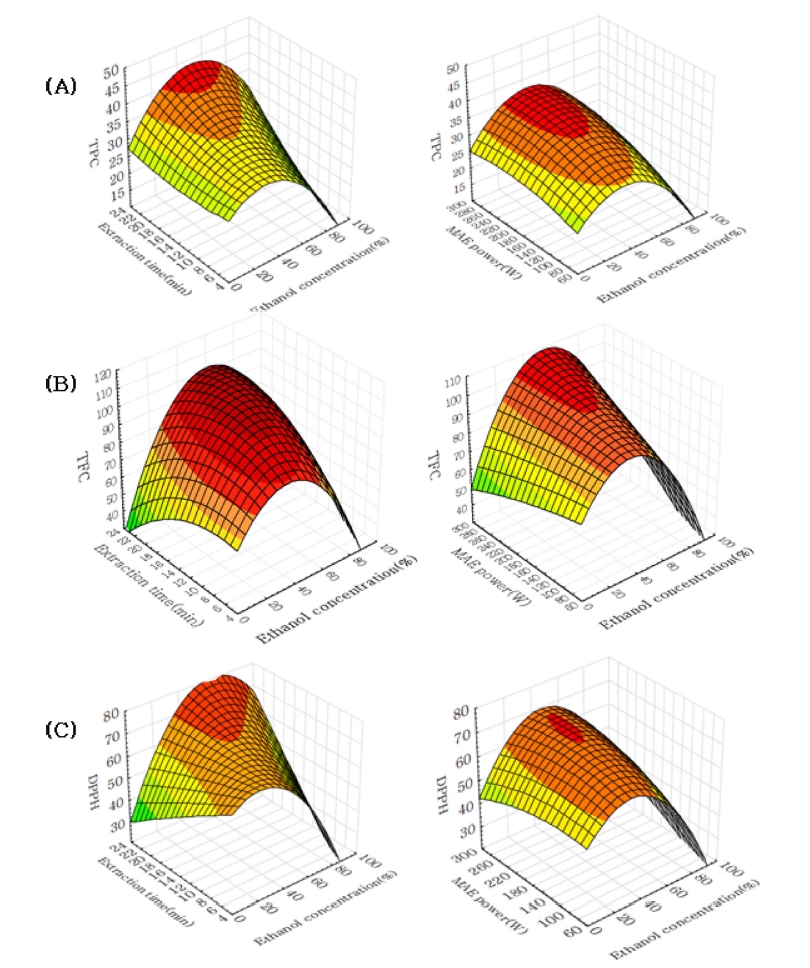
Surface plot for total polyphenol content (A), total flavonoid content (B) and DPPH radical scavenging activity (C) of ethanol concentration, microwave power and extraction time by microwave extraction.
In this study, aqueous ethanol shows a higher TPC than pure ethanol and water. This result indicated that aqueous ethanol reacts more effectively with microwave energy to produce a high TPC.
3. Effects of extraction condition on total flavonoid content (TFC)
The experimental result for TFC is shown in Table 2. The coefficient of determination for the polynomial equation was 0.9070, and significance was confirmed within a significance level of 0.05%. Fitted model for TFC is shown below:
The stationary point was the saddle point in RSM, and the predicted value of TFC was 108.72㎎• RE/g under an ethanol concentration of 48.09%, microwave power of 192.73W, and extraction time of 13.65 min (Table 3). Table 4 shows that ethanol concentration has a strong influence on the extraction of TFC. It satisfied a less than 0.05 significance level. The extraction time also show significant level within 0.1%, while it was confirmed that microwave power had insignificant effects. Fig. 2 shows that aqueous ethanol obtained higher flavonoid content than pure ethanol. A similar result was also indicated by Chen et al. (2008) in the extraction of Herba Epimedii. They observed that some water improves the extraction efficiency of flavonoids from Herba Epimedii.
In this study, extraction of TFC was enhanced in the range of ethanol concentration from 45 to 55% with increase of extraction time. But TFC was decreased more than 20min of extraction time. It could be presumed that, when the extraction time extend, the disintegration of bioactive substance may occur in extraction process. Therethrough it lead to a decline of extraction yield (Wang et al., 2008).
4. Effects of extraction condition on DPPH radical scavenging activity (DPPH)
Table 2 shows the experimental data of DPPH under various extraction conditions. The polynomial equation for DPPH shows the coefficient of determination is 0.9003 with a significance of less than 0.05%. Fitted model for DPPH is presented below:
The stationary point was the saddle point in RSM. Under the MAE conditions of 42.24% ethanol concentration, 216.17W of microwave power, and 11.92min of extraction time, the predicted value of DPPH was 71.74% (Table 3). Fig. 2 show that DPPH was risen within the range of ethanol concentration from 35 to 45% with increase of extraction time, but DPPH was decreased when the microwave power was over 280W. Among the extract conditions, ethanol concentration had a decisive effect, whereas microwave power and extraction time had lowimpact on the extraction of DPPH (Table 4). A similar result was formerly identified in the microwave-assisted extraction of Ligularia fischeri. Microwave power and extraction time had no significance for the variance of DPPH (Kwon et al., 2002).
5. Prediction of optimal extraction conditions and verification experiments
The optimal ranges for the extraction conditions of A. pilosa were predicted by superimposing a contour map. When ethanol concentration and microwave power were selected for superimposing, optimum conditions are 42 - 48%, 240 - 280W. And contour map of ethanol concentration and extraction time presented optimal conditions 40 - 60%, 14 - 20 min (Fig. 3), respectively. In accordance with the superimposing graph, the optimum conditions of ethanol concentration, microwave power, and extraction time were determined as 42 - 48%, 240 - 280W, and 14 - 20 min, respectively. A verification experiment was performed in the conditions of an ethanol concentration of 45%, microwave power of 270W, and extraction time of 14 min. Predicted values of TPC (36.82㎎• GAE/g), TFC (102.83㎎• RE/g), and DPPH (69.09%) were obtained through the polynomial equation. The experimental values for TPC, TFC, and DPPH were 35.15 ± 1.58㎎• GAE/g, 103.61 ± 4.36㎎• RE/g, and 68.74 ± 1.40%, respectively (Table 5).
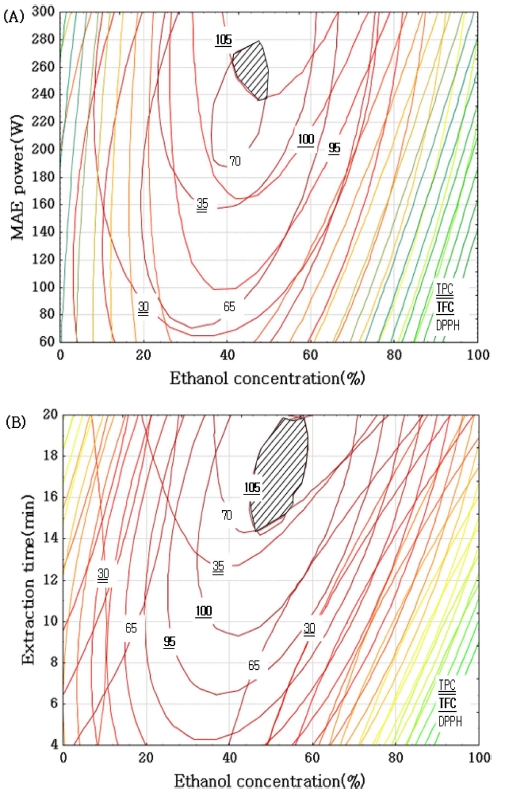
Superimposing contour map of optimized conditions for the total polyphenol, total flavonoid and DPPH radical scavenging activity of ethanol concentration and microwave power and extraction time by microwave extraction.
References
- Bae, JH, Sohn, MA, Effect ofAgrimonia pilosaLedeb extract on the growth of food-borne pathogens, The Korean Journal of Nutrition, (2005), 38, p112-116.
-
Blois, MS, Antioxidant determination by the use of a stable free radical, Nature, (1958), 181, p1199-1200.
[https://doi.org/10.1038/1811199a0]

-
Chen, L, Jin, H, Ding, L, Zhang, H, Li, J, Qu, C, Zhang, H, Dynamic microwave-assisted extraction of flavonoids from Herba Epimedii, Separation and Purification Technology, (2008), 59, p50-57.
[https://doi.org/10.1016/j.seppur.2007.05.025]

- Gao, Y, Zhou, G, Chen, J, Qin, H, Determination of six flavonoids inAgrimonia pilosaLedeb by HPLC, Food Science, (2015), 36, p93-96.
- Giese, J, Advances in microwave food processing, Food Technology, (1992), 46, p118-123.
- Kim, DH, An, BJ, Lee, JY, Whitening activities of theAgrimonia pilosaL extracts, Journal of Applied Biological Chemistry, (2011a), 54, p284-289.
- Kim, TH, Kim, JM, Baek, JM, Kim, TW, Kim, DJ, Park, JH, Choe, M, Antioxidant and whitening effects ofAgrimonia pilosaLedeb water extract, Korean Journal of Medicinal Crop Science, (2011b), 19, p177-184.
- Kim, YM, The inhibitory effect ofAgrimonia pilosaLedeb extract on allergic reaction, Korean Journal of Medicinal Crop Science, (2010), 8, p398-404.
- Kwon, JH, Kim, KG, Lee, GD, Optimization of microwave-assisted extraction under atmospheric pressure condition for soluble ginseng components, Korean Journal of Food Science and Technology, (2000), 32, p117-124.
- Kwon, MC, Kim, CH, Kim, HS, Lee, SH, Chio, GP, Park, UY, You, SG, Lee, HY, Optimal extract condition for the enhancement of anticancer activities of Artemisia princeps Pampanini, Korean Journal of Medicinal Crop Science, (2007), 15, p233-240.
- Kwon, YJ, Kim, KH, Kim, HK, Changes of total polyphenol content and antioxidant activity of Ligularia fischeri extracts with different microwave-assisted extraction conditions, Korean Journal of Food Preservation, (2002), 9, p332-337.
-
Lee, HJ, Do, Jr, Kwon, JH, Kim, HK, Optimization of Corni fructus extracts by response surface methodology, Journal of the Korean Society of Food Science and Nutrition, (2012), 41, p390-395.
[https://doi.org/10.3746/jkfn.2012.41.3.390]

- Lee, WY, Choi, SY, Lee, BS, Park, JS, Kim, MJ, Oh, SL, Optimization of extraction conditions from Omija (Schizandra chinensis Baillon) by response surface methodology, Korean Journal of Food Preservation, (2006), 13, p252-258.
- Min, KJ, Song, JW, Cha, CG, The antioxidative and antitumor activity of extracts ofAgrimonia pilosa, Journal of Food Hygiene and Safety, (2008), 23, p149-156.
- Pare, JRJ, Belanger, JMR, Stafford, SS, Microwaveassisted process(MAP™): A new tool for the analytical laboratory, Trends in Analytical Chemistry, (1994), 13, p176-184.
- Pei, YH, Li, X, Zhu, TR, Studies on the structure of a new ellagic acid glycoside from the root sprouts ofAgrimonia pilosaLedeb, Acta Pharmaceutica Sinica, (1990), 25, p798-800.
- Schiffmann, RF, Microwave processing in the US food industry Dielectric and ohmic sterilization, Food Technology, (1992), 46, p50-56.
- Singleton, VL, Orthofer, R, Lamuela-Raventos, RM, Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent, Methods in Enzymology, (1999), 299, p152-178.
-
Teng, H, Ghafoor, K, Choi, YH, Optimization of microwave-assisted extraction of active components from Chinese quince using response surface methodology, Journal of Applied Biological Chemistry, (2009), 52, p694-701.
[https://doi.org/10.3839/jksabc.2009.115]

- Théodore, K, Panda, T, Application of response surface methodology to evaluate the influence of temperature and initial pH on the production of ß-1,3-glucanase and carboxymethylcellulase from Trichoderma harzianum, Enzyme and Microbial Technology, (1995), 17, p1043-1049.
-
Wang, L, Li, D, Bao, C, You, J, Wang, Z, Shi, Y, Zhang, H, Ultrasonic extraction and separation of anthraquinones fromRheum palmatumL, Ultrasonics Sonochemistry, (2008), 15, p738-746.
[https://doi.org/10.1016/j.ultsonch.2007.12.008]

- Yoon, JY, Lee, SY, Jun, HJ, Lee, JY, Anti-aging effects of solvent fraction fromAgrimonia pilosaL extracts, Journal of Applied Biological Chemistry, (2012), 55, p35-39.
-
Zhang, HF, Yang, XH, Wang, Y, Microwave assisted extraction of secondary metabolites from plants Current status and future directions, Trends in Food Science and Technology, (2011), 22, p672-688.
[https://doi.org/10.1016/j.tifs.2011.07.003]
