
Effect of Controlled Light Environment on the Growth and Ginsenoside Content of Panax ginseng C. A. Meyer
© The Korean Society of Medicinal Crop Science. All rights reserved.
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License ( http://creativecommons.org/licenses/by-nc/3.0 ) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The photosynthetic efficiency cool-season, semi-shade ginseng is normal at low morning temperatures, but drops at high afternoon temperatures. Therefore, optimal plant performance would be ensured if it were possible to control daily light transmission rates (LTR).
Plants were grown in a controlled light environment that replicated 11 AM conditions and comparatively analyzed against plant grown under normal conditions. Growth in the controlled light environment resulted in a 2.81 fold increase in photosynthetic efficiency with no change in chlorophyll content, although LTR were high due to low morning temperatures. Increased aerial plant growth was observed in the ginseng plants adapted to the controlled light environment, which in turn influenced root weight. An 81% increase in fresh root weight (33.3 g per plant on average) was observed in 4-year-old ginseng plants grown in controlled light environment compared to the plants grown following conventional practices (18.4 g per plant on average). With regard to the inorganic composition of leaves of 4-year-old ginseng plants grown in controlled light environment, an increased in Fe content was observed, while Mn and Zn content decreased, and total ginsenoside content of roots increased 2.37 fold.
Growth of ginseng under a favorable light environment, such as the condition which exist naturally at 11 AM and are suitable for the plant’s photosynthetic activity creates the possibility of large scale production, excellent-quality ginseng.
Keywords:
Panax ginseng C. A. Meyer, Controlled Light Environment, Shading Facility, Light Transmission Rate, Photosynthetic EfficiencyINTRODUCTION
Ginseng (Panax ginseng C. A. Meyer) does not have stomas on the leaf surface; adaxial, but they are scattered across the backside; abaxial, and their number is very small. In addition, ginseng is one of the semi-shade plants whose chlorophyll development and Rubisco activation are low and whose chlorophyll-protein complex is created slowly, so the CO2 gas interchange quantity is very small (Yang et al., 1987; Xu and Zhou, 2008).
For this reason, for the ginseng leaves to be less stressed out, and for the optimal photosynthetic speed to be maintained, the light environment condition of 8 - 21 klux at 18 - 21℃ should be maintained (Lee, 1988). Since Korea has a big temperature difference according to season and time zone, and the light-blocking net is fixed in the case of a conventional shading facility, however, it is practically difficult to attract the proper amount of light. Most farmers ordinarily try to install only four-layer shading net and customarily add a two-layer shading net during summer with high temperature to lower the amount of light and temperature (RDA, 2009). As the temperature increases, the light saturation point falls, going down below 8 klux at over 35℃, with photo-oxidation proceeding quickly. Thus, the photosynthetic vitality of leaves drops (Lee et al., 2010).
If we look at the photosynthetic speed of ginseng by time zone during the day as measured in Korea, it is high until 11 AM when the temperature is around 23℃ with the sun, becoming very low as the temperature went up over 30℃ after that (Lee et al., 2012; Hyun et al., 1993). If high radiation intensity continues in the morning when the light saturation point becomes low as the temperature becomes high soon, the unnecessary light energy stimulates the leaves; consequently, single oxygen (1O2), superoxide (O2−), hydrogen peroxide (H2O2), and hydroxyl radical (−OH) occur excessively, causing the lipid components of the biological membrane to undergo peroxidation, which destroys the enzyme protein and chlorophyll rapidly (Yang et al., 1990; Ahn et al., 1994).
Damaged leaves cannot recover, so they fall early in the middle of August; in worse cases, root hypertrophy remarkably declines, and defoliation occurs (Mo et al., 2015). On the other hand, if we can control LTR every day, we will be able to draw an ideal result in terms of ginseng growth through the inflow of a lot of light at the beginning of 11 AM as the inflection point of photosynthetic efficiency and by drawing in less light later.
Therefore, this study was conducted to install sun shading that could control the light environment based on 11 AM and examine ginseng's growth and photosynthetic characteristics. It compared/analyzed the amount of ginsenosides as the representative second metabolite of ginseng to provide basic data for the study on improving ginseng’s physiology and sun shading facility.
MATERIALS AND METHODS
1. Operation of light environment control shade facility and growth condition
The experiments were performed in the experimental field at the department of Herbal Crop Research located in Eumseong, Chungbuk from March 28, 2014 to October 15, 2015. Pipes with diameter of 48㎜ were inserted into the ground at a depth of 0.6 m, and height measurement was adjusted to 2.5 m; dousers with width of 30㎝ were installed in blind form to control LTR. The ceiling was opened in the morning and closed after that at 11 AM every day. Of course, the ceiling was closed in the morning when it rained to prevent the occurrence of spread of disease such as alternaria blight by Alternaria panax, anthracnose by Colletotrichum gloeosporioides, and phytophthora blight by Phytophthora cactorum (Park et al., 2012).
Four- and two-black layer polyethylene shading nets were put on the timber A-type slant shade facility as a control plot. The test areas were 121㎡ for each treatment plot, and sudan grasses were sowed at the beginning of May 2013, harvested in the middle of July, and plowed up to 12 times at a 10-day interval with organic compost of 1,500㎏/10 a to manage the preparation field. 2-year-old violet-stemmed ginseng (2.5 ± 0.2 g) was planted at 72 plants/3.3㎡ in the east/west furrow direction, and disease/ pest prevention, weed removal, and other management were done based on the ginseng GAP standard cultivation guideline (RDA, 2009).
The external and internal radiation intensities of facilities were measured for 2 minutes and 3 times by LI-250A quantum sensor (LI-COR, Lincoln, NE, USA) at the height of the leaves to establish LTR following the light environment control; a thermometer (Thermo Recorder TR- 72Ui, T&D Corp., Tokyo, Japan) with data save function was hung around the leaves during the high-temperature period from July to August 2014 to measure the temperature.
2. Growth research and photosynthetic characteristics
The growth of the aboveground part was surveyed on July 21, 2014 and July 20, 2015; the underground part growth was researched for 30 weeks at random when all leaves fell by years. The leaf area was measured by the WindDIAS image analysis system (Delta-T Devices Ltd., Cambridge, England), length of stem and root, by general ruler, diameter of stem and root, by Digimatic calipers (Caliper 500-182, Mitutoyo, Kawasaki, Japan), and fresh root weight, by electronic scale (RE260, CAS, Seoul, Korea).
Data on chlorophyll content was printed out by putting the chlorophyll meter (SPAD-502, Konica Minolta, Tokyo, Japan) at the center of the leaf three times before harvesting the ginseng (Markwell et al., 1995).
The net CO2 assimilation rate (An) and stomatal conductance (gs) were measured by putting the LI6400 potable photosynthesis system (LI-COR, Lincoln, NE, USA) to which a 6㎠ leaf chamber was attached at the center of the leaf vertically at 9:00 AM and 3:00 PM on July 22, 2014. The internal conditions of the leaf chamber were set as follows: air influx rate of 500 μ㏖/s and CO2 concentration of 400 μ㏖. The temperature was opened in order to interlock with the outside (Lee et al., 2012).
3. Analysis of leaf inorganic component
3-year-old ginseng leaves were harvested on July 20, 2014, cleansed with distilled water, and freeze-dried for 72 hours at −80℃. They were decomposed to 0.5 g pulverized dry samples by using nitric acid, perchloric acid, and hydrochloric acid in that order and diluted and filtered to a fixed quantity. P2O5, K2O, MgO, CaO, Na2O, Cu, Mn, Fe, and Zn were quantified (NIAST, 2000) by the ICP analyzer (GBC Scientific Equipment, Braeside, Australia).
4. Analysis of root ginsenoside
10 kinds of ginsenosides were analyzed by finally harvesting 3-year-old and 4-year-old ginseng roots. The 10 kinds of ginsenoside standard samples were Rb1, Rb2, Rb3, Rc, Rd, Re, Rf, Rg1, Rg2, and Rh1 (ChromaDex, Irvine, CA, USA), the solvent for analysis was GR-grade. For the ginsenoside analysis, 0.2 g ginseng powder sample and 1㎖ of 70% MeOH were put into a 2㎖ tube to mix them well. The mixture was then put in ultrasonic bath for extraction by ultrasonic wave at 50℃ for 30 minutes. The supernatant obtained by centrifugal separation (4℃, 15 min) at 13,000 rpm was put into a 2㎖ tube, and 1㎖ was refined by the Sep-Pak C18 cartridge. The refined extract was filtered by a 0.45㎛ membrane filter for use as assay sample (Kim et al., 2008), and the ginsenoside content was measured using the Agilent 1100 series HPLC system (Agilent Technologies, Santa Clara, CA, USA). 10㎕ of the extract was injected using the Halo RPamide column (4.6 × 150㎜, 2.7㎛, Wilmington, DE, USA) and was analyzed at mobile flow velocity of 0.5㎖/min, column temperature of 50℃, and UV detector wavelength of 203㎚.
5. Statistical treatment
The results obtained from this experiment on the control group and treatment plot were analyzed via student's t-test using the SAS software package (SAS v9.2, SAS Institute Inc., Cary, NC, USA), and the difference was proven to be statistically significant when the p-value was under 0.05.
RESULTS AND DISCUSSION
1. Comparison of cultivation environment and photosynthetic characteristics
The LTR of the conventional shading facility in 2014 was 7.0%, which was in the optimal LTR 5 - 20% range for ginseng growth; it coincided with the light environment in the inclined facility installed by most farmers and researchers (Cheon et al., 1991a, b).
In controlling the light environment, LTR was 17.9% before 11 AM; the light inflow rate was higher than the conventional shading facility, but it did not exceed 20% and became 3.4% when the light was almost blocked after 11 AM (Table 1). The average temperature during the day at the time (6 AM - 8 PM) the sun was up from July 1 to August 31 was 1.2℃ lower than conventional practice (27.1℃) when the light environment (25.9℃) was controlled. This was because the hot air was not stagnant but escaped between the sun visors (Fig. 1). The lower temperature than the conventional shading facility and LTR control following the temperature rise had a positive effect on the morning photosynthetic efficiency of 3-year-old ginseng (Fig. 2). In the morning, An was 5.9 μ㏖/㎡/s, and gs was 0.08㏖/㎡/s; these were about 2.8 or 2 times higher than conventional practice, as the radiation intensity did not reach the light saturation point at a relatively low temperature than the afternoon.
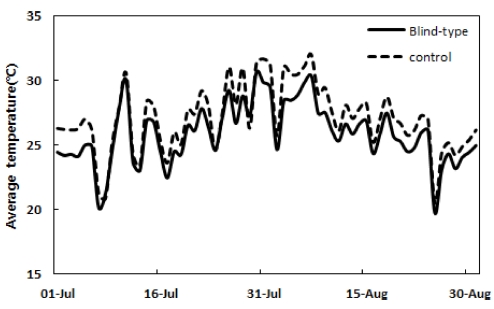
Average temperature at 6 AM - 8 PM of control and blind-type shading facilities from July 1, 2014 to August 31, 2014.
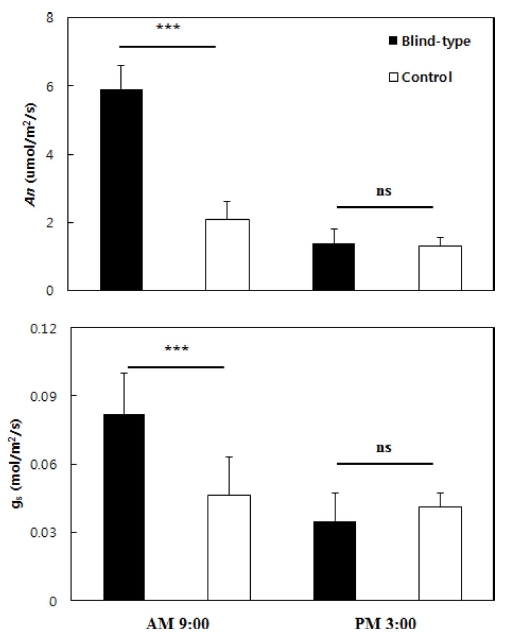
Net CO2 assimilation rate An and stomatal conductance gs of 3-year-old Panax ginseng grown in control and blind-type shading facilities at 9:00 AM and 3:00 PM on July 22, 2014.The vertical error bars represent the standard errors (n = 5). *p < 0.05, **p < 0.01, and ***p < 0.001 compared to the control. NS; Not significant.
Being put in a light environment wherein photosynthetic action could be carried out smoothly seemed to be the major reason unlike the control plot with limited An (Jochum et al., 2007). In addition, ginseng leaves exposed to strong light consistently or intermittently had high light adaptability, so An was formed more smoothly (Jang et al., 2015; Mo et al., 2015). This was because the photosynthetic efficiency in the morning was considerably different. Although the conventional facility’s LTR was more than 2 times higher in the afternoon, no statistical difference was found not only in An but also in gs due to the effect of the temperature rise.
The chlorophyll content of ginseng was higher in the 4- year-old one than in the 3-year-old one. This is because the formation of the leaves is ecologically stable, and mesophyll cells become thick in the 4 - 6-year-old aged roots (Fig. 3). In the ginseng leaves, the reduction of chlorophyll content by oxidized stress is distinct if light quantity exceeding the light saturation point enters (Yang et al., 1994). Although there is a difference according to the environment, if LTR exceeds 20%, this phenomenon becomes more serious because, if the leaves receive more light than the ones to be used, the response center of the light system II becomes inactive due to the photoinhibition phenomenon (Ort and Baker, 1988).
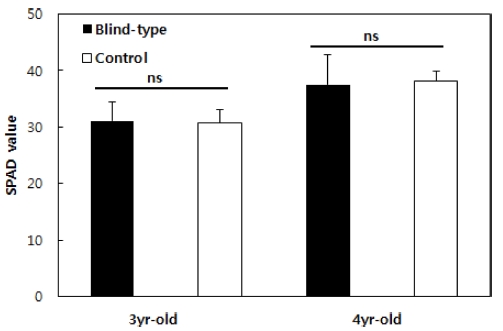
SPAD value of 3- and 4-year-old Panax ginseng grown in control and blind-type shading facilities on July 21, 2014 and July 20, 2015.The vertical error bars represent the standard errors (n = 30). *p < 0.05, **p < 0.01, and ***p < 0.001 compared to the control. NS; Not significant.
As seen in the data, however, although LTR was high in the morning when the light environment was controlled, there was no difference from the control in terms of chlorophyll content. Dynamic photo-inhibition is observed at moderate light. Although the quantum efficiency decreases, the photosynthetic speed does not change. The reason for the dynamic photo-inhibition is that the absorbed light energy is transferred to the heat dissipation, and quantum efficiency declines (Tyystjarvi et al., 1991). Since this reduction is temporary, if the photon flux drops below the saturation level, it can be restored to the original high value.
2. Growth of ginseng
There is a difference depending on the environment and kind. Proctor et al. (2010), who tested on P. quinquefolius, reported that, when LTR increased to 30%, the aboveground part and underground part growth increased. Kim et al. (1982) claimed that the ginseng that grew in the front row of the shading facility where relatively more light shone grew better than the ones that grew in the rear row. According to our test, where the light environment was controlled, the light influx in the morning could increase the aboveground part growth of ginseng compared to the control (Table 2).

Growth of aerial part of 3- and 4-year-old Panax ginseng grown in control and blind-type shading facilities on July 21, 2014 and July 20, 2015.
In particular, the area of the leaves as the light acceptor broadened by 34% on 3-year-old ginseng in 2014. The increase of leaf area and An in the morning led to the enhancement of the root, which was the storage of metabolite, affecting the growth of the 4-yearold ginseng the following year (2015) because the growth of ginseng that year is greatly influenced by the prolificacy of the previous-year ginseng. Jang et al. (2015) reported that the leaf area became broad when LTR increased up to 17% in the case of 2-year-old ginseng; this is because, when excessive light shone after the leaves became broad up to a certain level, the leaves diminished, recognizing it as stress.
In the end, the light environment regulation for 2 years based on 11 AM increased the root weight of the 4- year-old ginseng by about 81% compared to the control (Table 3). Meanwhile, as blind-type shading facility was continuously controlled after the end of the sweltered July, leaf burn of ginseng rapidly happened. It assumed that dry damage was also partially generated because of lowered soil and air humidity.
3. Analysis of leaf inorganic component and root ginsenoside
As shown in Table 4, P2O5, K2O, MgO, CaO, Na2O, and Cu had no statistically significant difference. Note, however, that there was a big difference in Mn, Fe, and Zn. Fe is a component of hemoprotein and Fe-S protein existing in the redox system, and about 80% exists in chlorophyll. Table 5

Inorganic component contents of 3-year-old Panax ginseng leaf grown in control and blind-type shading facilities on July 21, 2014.

Ginsenoside contents of roots of 3- and 4-year-old Panax ginseng grown in control and blind-type shading facilities on the final harvest day.
Since Fe is a component of aconitase, an enzyme that changes the citrate into insocitrate during the Krebs Cycle, if Fe is deficient, organic acid is accumulated, carbohydrate metabolism is affected, and redistribution does not occur in the plant; thus, the chlorophyll content of the young leaves is low, and the leaves become chlorotic (Jeong and Guerinot, 2009). Although the chloroplast source of Fe is not well-known with regard to the synthesis of the photosynthetic cofactors, ferritin, an Fesaving protein, will be relevant because the buffer action such as saving Fe is done to avoid excessive oxidative stress (Yruela, 2009).
Mn and Zn are indispensable mineral elements in photosynthesis in association with the superoxide dismutase; according to our experiment, it seems to have decreased somewhat as the benefit in return, i.e., Fe accumulated on the leaves (Armstrong, 2008).
Well-known as one of the medicinal properties, ginsenoside acts as the indicator that evaluates the quality of ginseng. Ginseng that is inevitably exposed to various kinds of stress for years or dozens of years in one place produces the saponin ingredients for the purpose of protecting itself (Choi et al., 2005).
In this experiment, which controlled the light environment, the amount of ginsenoside consisting of 10 kinds Rb1, Rb2, Rb3, Rc, Rd, Re, Rf, Rg1, Rg2, and Rh1 increased regardless of life in years, although the biomass of ginseng increased. The ginsenoside content increased more in the 4- year-old ginseng than in the 3-year-old ginseng. When the light environment was controlled, the increase range (36%) was 13% higher than the control (23%). Although the study result was opposite to the one (Han et al., 2013) stating that the ginsenoside content was low when the ginseng was heavy in conventional cultivation, light stress is considered one of the environment factors wielding a big effect on the ginsenoside content increase of ginseng (Jang et al., 2015).
In sum, light environment control before and after 11 AM could increase the photosynthetic efficiency without a change in chlorophyll content, although LTR was high because of the low temperature in the morning. The aboveground part growth of the ginseng that adapted to the light environment increased to affect the root weight, and the total ginsenoside content of the 4-year-old ginseng multiplied by 2.37 times compared to the control due to proper light stress. Therefore, if light environment management according to the photosynthetic nature of the ginseng is carried out, it will be possible to produce large-capacity, excellent-quality ginseng.
ACKNOWLEDGMENTS
This work was carried out with the support of Cooperative Research Program for Agriculture Science and Technology Development(PJ01098502) Rural Development Administration, Republic of Korea.
References
- Ahn, JS, Cho, BG, Park, H, Kim, WK, Changes in chloroplast ultrastructure and thylakoid membrane proteins by high light in ginseng leaves, Journal of Plant Biology, (1994), 37, p285-292.
-
Armstrong, FA, Why did nature choose manganese to make oxygen?, Philosophical Transactions of the Royal Society B: Biological Sciences, (2008), 363, p1263-1270.
[https://doi.org/10.1098/rstb.2007.2223]

- Cheon, SK, Mok, SK, Lee, SS, Effects of light intensity and quality on the growth and quality of Korean ginseng(Panax ginsengC A Meyer): II Relationship between light intensity and planting density, Korean Journal of Ginseng Science, (1991b), 15, p31-35.
- Cheon, SK, Mok, SK, Lee, SS, Shin, DY, Effects of light intensity and quality on the growth and quality of Korean ginseng(Panax ginsengC A Meyer): I Effects of light intensity on the growth and yield of ginseng plants, Korean Journal of Ginseng Science, (1991a), 15, p21-30.
-
Choi, DW, Jung, JD, Ha, YI, Park, HW, In, DS, Chung, HJ, Liu, Jr, Analysis of transcripts in methyl jasmonatetreated ginseng hairy roots to identify genes involved in the biosynthesis of ginsenosides and other secondary metabolites, Plant Cell Report, (2005), 23, p557-566.
[https://doi.org/10.1007/s00299-004-0845-4]

- Han, JS, Tak, HS, Lee, GS, Kim, JS, Woo, RJ, Choi, JE, Comparison of ginsenoside content and ratio of root tissue according to root age and diameter inPanax ginsengC A Meyer, Korean Journal of Medicinal Crop Science, (2013), 21, p342-347.
- Hyun, DY, Hwang, JK, Choi, SY, Jo, JS, Photosynthetic characteristics ofPanax ginsengC A Meyer I Photosynthetic response to changes of light intensity and leaf temperature, Korean Journal of Ginseng Science, (1993), 17, p240-245.
-
Jang, IB, Lee, DY, Yu, J, Park, HW, Mo, HS, Park, KC, Hyun, DY, Lee, EH, Kim, KH, Oh, CS, Photosynthesis rates, growth, and ginsenoside contents of 2-yr-oldPanax ginsenggrown at different light transmission rates in a greenhouse, Journal of Ginseng Research, (2015), 39, p345-353.
[https://doi.org/10.1016/j.jgr.2015.03.007]

-
Jeong, J, Guerinot, ML, Homing in on iron homeostasis in plants, Trends in Plant Science, (2009), 14, p280-285.
[https://doi.org/10.1016/j.tplants.2009.02.006]

- Jochum, GM, Mudge, KW, Thomas, RB, Elevated temperatures increase leaf senescence and root secondary metabolite concentrations in the understory herbPanax quinquefolius(Araliaceae), American Journal of Botany, (2007), 94, p819-826.
- Kim, GS, Hyun, DY, Kim, YO, Lee, SE, Kim, YC, Lee, SW, Son, WD, Lee, MJ, Park, CB, Park, HK, Cha, SW, Song, KS, Extraction and preprocessing methods for ginsenosides analysis ofPanax ginsengC A Meyer, Korean Journal of Medicinal Crop Science, (2008), 16, p446-454.
- Kim, JM, Lee, SS, Cheon, SR, Cheon, SK, Relationship between environmental conditions and the growth of ginseng plant in field: I. Productive structures as affected by planting positions and ages, Korean Journal of Crop Science, (1982), 27, p94-98.
- Lee, CH, Effect of light intensity and temperature on the photosynthesis and respiration of Panax spp, Korean Journal of Ginseng Science, (1988), 12, p11-29.
-
Lee, JS, Lee, DY, Lee, JH, Ahn, IO, In, JG, Photosynthetic characteristics of resistance and susceptible lines to high temperature injury inPanax ginsengMeyer, Journal of Ginseng Research, (2012), 36, p461-468.
[https://doi.org/10.5142/jgr.2012.36.4.461]

- Lee, JS, Lee, JH, Ahn, IO, Characteristics of resistant lines to high-temperature injury in ginseng(Panax ginsengC A Meyer), Journal of Ginseng Research, (2010), 34, p274-281.
-
Markwell, J, Osterman, JC, Mitchell, JL, Calibration of the Minolta SPAD-502 leaf chlorophyll meter, Photosynthesis Research, (1995), 46, p467-472.
[https://doi.org/10.1007/bf00032301]

-
Mo, HS, Jang, IB, Yu, J, Park, HW, Park, KC, Effects of enhanced light transmission rate during the early growth stage on plant growth, photosynthetic ability and disease incidence of above ground inPanax ginseng, Korean Journal of Medicinal Crop Science, (2015), 23, p284-291.
[https://doi.org/10.7783/kjmcs.2015.23.4.284]

- National Institute of Agricultural Sciences and Technology (NIAST), Methods of soil chemical analysis, (2000), Rural Development Administration, Suwon, Korea, p108-149.
- Ort, DR, Baker, NR, Consideration of photosynthetic efficiency at low light as a major determinant of crop photosynthetic performance Quantum yield, coupling factor, electrocheminal potential, antenna chlorophyll complexes, Plant Physiology and Biochemistry, (1988), 26, p555-565.
-
Park, YH, Lee, SG, Ahn, DJ, Kwon, TR, Park, SU, Lim, HS, Bae, H, Diversity of fungal endophytes in various tissues ofPanax ginsengMeyer cultivated in Korea, Journal of Ginseng Research, (2012), 36, p211-217.
[https://doi.org/10.5142/jgr.2012.36.2.211]

- Proctor, JTA, Palmer, JW, Follett, JM, Growth, dry matter partitioning, photosynthesis in North American ginseng seedlings, Journal of Ginseng Research, (2010), 34, p175-182.
- Rural Development Administration(RDA), Ginseng standard cultivation textbook, (2009), Rural Development Administration, Suwon, Korea, p47-139.
-
Tyystjarvi, E, Koivuniemi, A, Kettunen, R, Aro, EM, Small light-harvesting antenna does not protect from photoinhibition, Plant Physiology, (1991), 97, p477-483.
[https://doi.org/10.1104/pp.97.2.477]

-
Xu, Z, Zhou, G, Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass, Journal of Experimental Botany, (2008), 59, p3317-3325.
[https://doi.org/10.1093/jxb/ern185]

- Yang, DC, Chae, Q, Lee, SJ, Kim, YH, Kang, YH, Effects of light and photosynthetic electron transport system on the generation of singlet oxygen(1O2):in ginseng thylakoid membrane, Korean Journal of Ginseng Science, (1990), 14, p57-62.
- Yang, DC, Kim, YH, Yang, DC, Hong, YN, Effects of antioxidants on the photosynthesis and carbohydrates/saponin contents inPanax ginsengleaves, Korean Journal of Ginseng Science, (1994), 18, p175-181.
- Yang, DC, Yoo, HS, Yoon, JJ, Investigation on the photooxidation of pigment in leaf-burning disease ofPanax ginseng: II. Investigation and analysis of physiological reaction mechanism on the chlorophyll bleaching phenomenon, Korean Journal of Ginseng Science, (1987), 11, p101-110.
-
Yruela, I, Copper in plants Acquisition, transport and interactions, Functional Plant Biology, (2009), 36, p409-430.
[https://doi.org/10.1071/fp08288]
