
Effect of Valeriana fauriei Extract on the Neurodevelopmental Proteins Expression and Behavioral Patterns in Maternal Immune Activation Animal Model
†Corresponding author: +82-41-570-2424 hak3962@sch.ac.kr
© The Korean Society of Medicinal Crop Science. All rights reserved.
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License ( http://creativecommons.org/licenses/by-nc/3.0 ) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Prenatal exposure to infectious and/or inflammatory insults can increase the risk of developing neuropsychiatric disorder such as bipolar disorder, autism, and schizophrenia later in life. We investigated whether Valeriana fauriei (VF) treatment alleviates prepulse inhibition (PPI) deficits and social interaction impairment induced by maternal immune activation (MIA).
Pregnant mice were exposed to polyriboinosinic-polyribocytidilic acid (5㎎/㎏, viral infection mimic) on gestational day 9. The adolescent offspring received daily oral treatment with VF (100㎎/㎏) and injections of clozapine (5㎎/㎏) for 30 days starting on the postnatal day 35. The effects of VF extract treatment on behavioral activity impairment and protein expression were investigated using the PPI analysis, forced swim test (FST), open field test (OFT), social interaction test (SIT), and immunohistochemistry. The MIA-induced offspring showed deficits in the PPI, FST, OFT, and SIT compared to their non MIAinduced counterparts. Treatment with the VF extract significantly recovered the sensorimotor gating deficits and partially recovered the aggressive behavior observed in the SIT. The VF extract also reversed the downregulation of protein expression induced by MIA in the medial prefrontal cortex.
Our results provide initial evidence of the fact that the VF extract could reverse MIA-induced behavioral impairment and prevent neurodevelopmental disorders such as schizophrenia.
Keywords:
Valeriana fauriei Briq., Maternal Immune Activation, Polyriboinosinic-Polyribocytidilic Acid, SchizophreniaINTRODUCTION
Valeriana fauriei Briq. (VF) has been used in humans for hundreds of years (Liu et al., 2012). This genus with over 250 species contains a variety of compounds, including valepotriates, valerenic acid, and its derivatives (Ahn et al., 2012; Liu et al., 2012). Valeriana has been used for people many years in China and Korea (Lee et al., 2016b).
Valeriana genus has been used for centuries for treating epilepsy, myalgia, sleep disorders, and anxiety (Bent et al., 2006). Many studies have shown its neuroprotective effect in in vitro models of Parkinson’s disease. An aqueous extract of Valeriana has significant cytoprotective effect on rotenone-induced apoptosis in human neuroblastoma SHSY5Y cells (de Oliveria et al., 2009). Its antiinflammatory properties as an inhibitor of NF-κB has emerged from the knowledge of its traditional use as an anti-inflammatory remedy in Europe (Jacobo-Herrera et al., 2006).
Miyasaka et al. (2006) have reported the efficacy and safety of valerian as a treatment option for anxiety disorders. In previous studies, the effects of VF on prenatal stress (PNS) offspring-related psychiatric disorders such as depression and schizophrenia were determined (Kang et al., 2014; Lee et al., 2016a). In addition, changes in protein levels and behavioral patterns were examined in the prefrontal cortex of PNS rats. We have revealed that the changes due to PNS are affected by VF treatment (Lee et al., 2016a). Considering these data and the already known effects of VF on various diseases, we proposed that VF might be a target in the search for new agents to assist treatment for psychiatric disorders.
Therefore, the objective of this study was to determine the effect of VF administration on behavioral symptoms and the expression of neurodevelopmental proteins in a MIA model.
Maternal immune activation (MIA) due to infection during pregnancy has been repeatedly implicated in the etiology of developmental neuropsychiatric disorders, including bipolar disorder (Canetta et al., 2014), autism (Brown et al., 2014), and schizophrenia (Fatemi et al., 2008). MIA can be induced by injecting pregnant dams with viral mimic polyriboinosinicpolyribocytidilic acid (Poly I:C), leading to a wide spectrum of schizophreniarelevant functional and neuropathological deficits in adult offspring (Meyer et al., 2010b).
Many MIA-induced behavioral, cognitive, and pharmacological dysfunctions in adult offspring are directly implicated in schizophrenia and other psychosis-related disorders, including abnormalities in sensorimotor gating, selective attention, deficits in social interaction, working memory, and sensitivity to psychostimulant drugs (Bitanihirwe et al., 2010).
In schizophrenia-like animal model induced by MIA, chronic antipsychotic drug treatment during periadolescence may prevent subsequent emergence of psychosis-related behavioral and pharmacological abnormalities in adulthood (Meyer et al., 2010b). In addition, clozapine can effectively block phencyclidine-induced hyperlocomotion, improving the disruption of prepulse inhibition and deficits in social interaction (Bakshi et al., 1994).
MATERIALS AND METHODS
1. Preparation of Valeriana fauriei extracts
The VF was obtained from a local farm in Jingbu province Republic of Korea. The roots of VF were airdried avoiding sun-light and cut into small pieces for the experiment. The dried roots (2㎏) were soaked in 70% ethanol (3ℓ) at room temperature for 1 day and extracted for 1 hour three times with 70% EtOH in an ultrasonic apparatus and filtered with filter paper (Advantec Toyo Kaisha Ltd., Tokyo, Japan) to remove the debris. The EtOH extract was evaporated under reduced pressure by rotary evaporator and lyophilized with freezing dryer to give 70% EtOH crude extract (330.2 g, yield 16.5%).
2. Animals
C57BL6/J mice (8 weeks old) were purchased from Central Lab Animal Inc. (Seoul, Korea). Mice were mated in groups of 1 male and 2 females. When a vaginal plug was observed during daily control, female mice were considered pregnant and separated. All mice were housed under standard conditions of a 12/12-h light/dark cycle (lights on at 06 : 30) with free access to food and water. All animal procedures were performed in accordance with the guidelines for the care and use of laboratory animals provided by the US National Institutes of Health (NRC, 1996).
3. Maternal immune activation and drug administration to mice
Clozapine (5㎎/㎏/day, Sigma-Aldrich Co., St. Louis, MO, USA) was dissolved in acetic acid and diluted with saline followed by intraperitoneal injection. VF (100㎎/㎏/ day) was dissolved in water and orally administered on postnatal day 35 for 4 weeks until postnatal day 65 (Meyer et al., 2010b).
Pregnant dams on GD 9 received either a single injection of Poly I:C or CON (saline) solution via intravenous route at the tail vein under mild physical constraint. Poly I:C (potassium salt) was obtained from Sigma-Aldrich Co. (St. Louis, MO, USA) and dissolved in isotonic 0.9% NaCl solution to obtain the desired dosage (5㎎/㎏ calculated based on pure form of Poly I:C. All animals were returned to their home cages immediately after the injection procedure and left undisturbed until weaning of the offspring.
4. Behavioral tests
Modified behavioral tests, including forced-swim test (FST), open-field test (OFT), social interaction test (SIT), and prepulse inhibition (PPI) test, were performed as described previously (Lee et al., 2016a).
An automated startle reflex system (SR Lab, San Diego Instruments, San Diego, CA, USA) was used to measure prepulse inhibition. This system consisted of a startle chamber housed in a sound attenuated isolation cabinet equipped with an internal fan and light. A cylindrical transparent acrylic holding apparatus resting on a fourpegged platform within the isolation chamber was used to hold each subject throughout the testing session.
Background noise and acoustic stimuli were controlled via the SR Lab microcomputer and interface assembly. They were delivered through a speaker mounted above the cylindrical holding apparatus. All test chambers were located in a sound attenuated experimental room to minimize external noise (Chang et al., 2015). Background noise of 68 dB was present throughout the test session. After a 5min acclimation period to the background noise, trials were presented in pseudorandom order, including 14 pulse alone trials in which a 40 ms, 120 dB broadband noise burst was presented, 30 prepulse + pulse trials in which the onset of a 20 ms broadband noise prepulse preceded the onset of the 120 dB pulse by 100 ms (10 for each of prepulse intensities of 3, 6, and 12 dB above the background noise, respectively), and eight non-stimulus trials consisting of only background noise. Prepulse intensities used in our protocol did not induce startle reaction. All trials were presented with an average inter-trial interval of 22 s (15 - 30 s range). Four 120 dB pulse trials were presented at the beginning and the end of the test session with a series of 60 acoustic stimuli trials. However, they were not used in the calculation of PPI values.
The holding chambers were cleaned with 75% ethanol between each test session. The level of PPI was calculated as a percentage score for each prepulse using the following formula: %PPI = 100 - [{(startle response for prepulse + pulse trial) / (startle response for pulse alone trial)} × 100] (Nozari et al., 2015). Mean %PPI was considered as an overall measure of the observed treatment for which percent PPI data were averaged for three prepulses (Meyer et al., 2010b).
FST was conducted as described previously (Adachi et al., 2008). Mice were gently placed in a large transparent cylinder filled with fresh warm tap water (25 ± 2℃) for 5min. The water was changed between mice. The behaviors of swimming, climbing, and immobility were recorded with a video camera by an observer with a stopwatch.
OFT was used to assess exploratory activity and reactivity to a novel environment. The test was carried out in clear opaque Plexiglas boxes (50㎝ × 50㎝ × 25㎝) equipped with a video as described previously (Cryan and Mombereau, 2004). Mice were placed in the center of the apparatus and their locomotor behaviors were recorded for 20 min. Horizontal locomotor activity was expressed as total ambulatory distance. The test box was cleaned with 70% ethanol between tests.
SIT was adapted from previous studies (Zhu et al., 2014). Using the same open-field box, before the experiment, mice were placed in the environment for 30 min for acclimatization. SIT is commonly referred to as the behavior that occurs in a social context resulting from interaction between and among individuals (of the same species with equal body weight).
The behaviors of mice were recorded by a video camera placed above the arena. Social interaction included the following behaviors; following or approaching the test partner, mounting or biting the test partner, sniffing or grooming any part of the body of the test partner. Each session lasted 20 min. Total duration of social play and the numbers and types of interactions were recorded.
5. Western blot
Medial prefrontal cortex (mPFC) tissues were lysed in RIPA buffer containing protease inhibitors followed by centrifugation at 14,000 rpm for 10 min at 4℃.
To detect dihydropyrimidinase-like 2 (Dpysl2) and neurofilament protein, 40 g of lysed protein was subjected to 10 and 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (Merck Millipore, Billerica, MA, USA). After blocking with 5% skim milk, membranes were probed with anti-Dpysl2 (1 : 1,000, Cell Signaling Technology Inc., Danvers, MA, USA), anti-LIM and SH protein 1 (Lasp1, 1 : 2,000, Merck Millipore, Billerica, MA, USA), antineurofilament M (Nefm, 1 : 1,000, Cell Signaling Technology Inc., Danvers, MA, USA), or anti-β-tubulin (Tubb, 1 : 3,000, Thermo Fisher Scientific Inc., Waltham, MA, USA) antibodies overnight at 4℃. After washing three times, membranes were then incubated with peroxidase-conjugated secondary anti-mouse (1 : 10,000, Sigma-Aldrich Co., St. Louis, MO, USA) or anti-rabbit (1 : 5,000, Abfrontier, Young In Frontier Co., Ltd., Seoul, Korea) for 1 h at room temperature. Immunoreactive bands were detected using an enhanced chemiluminescence kit (ELPIS-Biotech Inc., Daejeon, Korea). Quantitative measurements of Dpysl2, Lasp1, Nefm and Actb proteins were obtained using ImageJ software.
6. Immunohistochemistry
Mice were deeply anesthetized with ethyl ether and perfused with 4% paraformaldehyde. Fixed brains were removed, frozen, and cut into 30㎛ sections. Frozen sections from mPFC were blocked with normal horse serum, incubated with anti-Dpysl2 (1 : 700, Atlas Antibodies AB, Stockholm, Sweden), Nefm (1 : 100, Cell Signaling Technology, Danvers, MA, USA), and anti-NeuN (1 : 100, Merck Millipore, Billerica, MA, USA) followed by incubation with Cy3- conjugated anti-rabbit and mouse secondary antibodies (1 : 500 and 1 : 800, Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA). Fluorescent images were captured using a confocal laser-scanning microscope (FV10-ASW, Olympus Co., Tokyo, Japan). Images were quantified with Image J software using a protocol described previously with slight modifications (Kim et al., 2015).
7. Statistical analysis
All data are expressed as mean ± standard deviation and/ or standard error of the mean. They were compared using Student’s t-test. All statistical analyses were performed using IBM SPSS statistics 22 software (SPSS Inc, Chicago, IL, USA). p-values < 0.05 were considered as statistically significant.
RESULTS AND DISCUSSION
We used mouse MIA model to evaluate the extent to which the VF treatment altered the behavioral and protein expression that might be related to the pathophysiology of MIA-induced psychiatric disorders due to Poly I:C during pregnancy. We investigated the effects of VF treatment on MIA-induced behavioral phenotypes with PPI, FST, OFT, and SIT.
1. Prepulse inhibition
The effects of clozapine and VF on MIA-induced PPI deficits in mice are shown in Fig. 1.
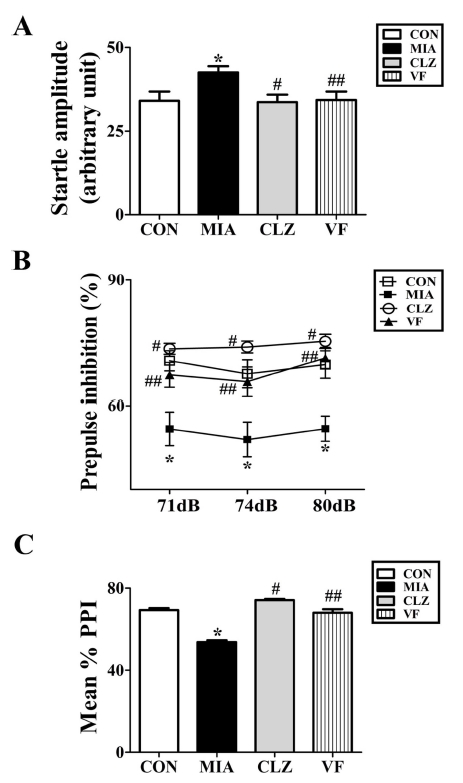
Prepulse inhibition (PPI) was analyzed in adult male mice exposed to MIA or male mice derived from litters without MIA exposure.PPI is presented as a mean present PPI value in startle amplitude as a function of the magnitude of prepulse stimulus using the following formula; mean present PPI = 100 - [{(startle response for prepulse + pulse trial) / (startle response for pulse alone trial)} × 100]. Data are presented as means ± SEM (*p < 0.05 compared to CON, #,##p < 0.05 compared to MIA). CON; offspring of non-MIA mice, MIA; offspring of MIA mice, CLZ; offspring of MIA mice treated with clozapine drug, VF; offspring of MIA mice treated with Valeriana fauriei extract.
We tested whether clozapine and VF treatment might be effective in preventing emergence of sensorimotor gating deficiency following MIA during adulthood. Sensorimotor gating was evaluated using the paradigm of PPI of acoustic startle reflex (ASR). Peripubertal clozapine and VF administration prevented the disruption of PPI in the offspring exposed to MIA (p < 0.05, Fig. 1). Clozapine and VF administration also decreased ASR levels in MIA offspring (p < 0.05, Fig. 1A).
Our results indicated that MIA significantly (p < 0.05) altered PPI level at prepulse stimulus levels of 6, 9, and 12 dB above background between MIA offspring and control offspring. VF treatment significantly (p < 0.05) elevated PPI trials scores in MIA offspring to levels found in control offspring (Fig. 1B). Poly I:C exposure to MIA affected (p < 0.05) the reactivity to mean percent PPI (Fig. 1C). These results suggest that clozapine and VF can prevent the development of sensorimotor gating deficiency in MIA offspring.
2. Forced swim test (FST)
We found significant differences in FST results among control, MIA, and VF-treated groups (Fig. 2). In particular, MIA offspring exhibited decrease in climbing but increase in immobility behaviors compared to control offspring (p < 0.05, Fig. 2). The changed behaviors were recovered after treatment with clozapine or VF (p < 0.05, Fig. 2).
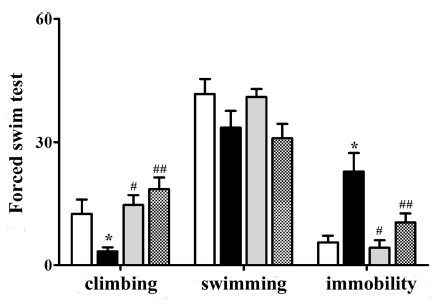
Behavioral response in forced-swim test.Comparison between offspring of the CON and MIA groups. A decrease in climbing activity was observed. Data are presented as means ± SEM (*p < 0.05 compared to CON, #,##p < 0.05 compared to MIA). CON; offspring of non-MIA mice, MIA; offspring of MIA mice, CLZ; offspring of MIA mice treated with clozapine drug, VF; offspring of MIA mice treated with Valeriana fauriei extract.
3. Open-field test (OFT)
Control and MIA offspring were subjected to OFT for 20 min. The MIA group had a significantly decrease in the number of line crossings and duration of rearing behaviors. These scores were recovered to their normal levels after treatment with clozapine or VF (p < 0.05, Table 1).

Behavior of CON and MIA and clozapine treatment of MIA and Valeriana fauriei extract treatment of MIA in an open field test.
The MIA group showed a significantly increase in the number of cage sniffing and immobility behaviors. These scores were also recovered to their normal level after treatment with clozapine or VF (p < 0.05, Table 1). However, the MIA offspring did not show significant difference in central entries, number of rearing behaviors, or the duration of cage sniffing compared to control offspring (p > 0.05, Table 1).
4. Social interaction test (SIT)
MIA also induced severe social deficits (Table 2). Scores of most aggressive behaviors (aggressive grooming the partner, biting the partner) during SIT were increased significantly in the MIA group compared to those in the control group. These scores were decreased to normal levels after treatment with clozapine or VF (p < 0.05, Table 2).

Behavior of CON and MIA and clozapine treatment of MIA and Valeriana fauriei extract treatment of MIA in a social interaction test.
On the other hand, reductions in sniffing the partner and grooming the partner induced by MIA were rescued by treatment with VF. These results indicate that VF can ameliorate defective social interactions in MIA-induced animal model (p < 0.05, Table 2).
5. Western blot and immunohistochemistry
Our previous studies have shown that Lasp1 and Dpysl2 are regulated in prefrontal cortex in schizophrenia-like animal model (Joo et al., 2013; Lee et al., 2015).
To investigate MIA-induced downregulation of several neurodevelopmental proteins such as Lasp1, Dpysl2, and Nefm proteins, we performed Western blotting and immunohistochemical analyses (Fig. 3, 4, 5) of the mPFC areas from control, MIA, clozapine, and VF administered offspring brains. Western blot results revealed that the quantities of these three proteins in the mPFC area of the MIA group were significantly lower than those in the control group (p < 0.05, Fig. 3). These changes were restored by clozapine or VF treatment (p < 0.05, Fig. 3). Immunofluorescent-stained brain images revealed that Dpysl2 and Nefm were differentially expressed among control, MIA, clozapine treated, and VF treated groups with significant differences in staining intensity value (p < 0.05, Fig. 4, 5).
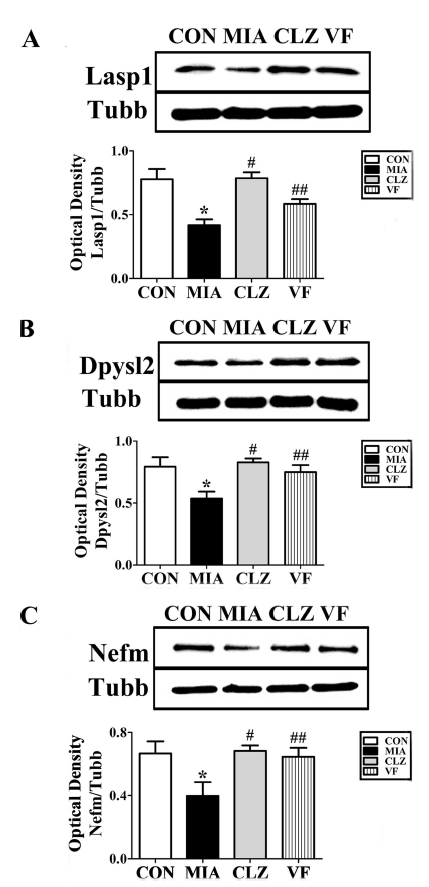
Western blot analysis of Dpysl2, Lasp1, and Nefm expression in the brains of MIA-induced mice.(A); mice exposed to maternal immune activation (MIA) exhibited decreased Lasp1 expression in the medial prefrontal cortex. Lasp1 expression showed a significant difference in Lasp1 levels between MIA treated and VF-treated groups. (B); mice exposed to maternal immune activation (MIA) exhibited decreased Dpysl2 expression in the medial prefrontal cortex. Dpysl2 expression showed significant difference in Dpysl2 levels between MIA treated and VF-treated groups. (C); mice exposed to maternal immune activation (MIA) exhibited decreased Nefm expression in the medial prefrontal cortex. Nefm expression showed significant difference in Nefm levels between MIA treated and VF-treated groups (*p<0.05 compared to the CON group in the medial prefrontal cortex, #,##p<0.05 compared to MIA group in the medial prefrontal cortex). CON; offspring of non- MIA mice, MIA; offspring of MIA mice, CLZ; offspring of MIA mice treated with clozapine drug, VF; offspring of MIA mice treated with Valeriana fauriei extract.
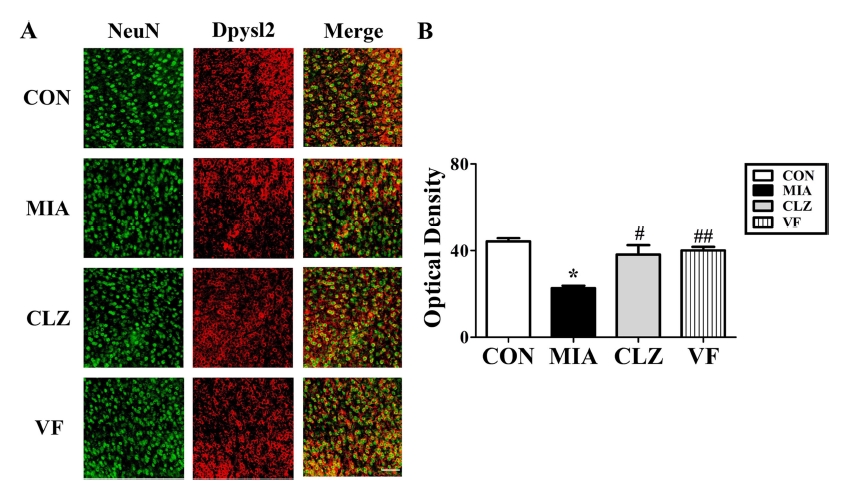
Immunohistochemical analysis of Dpysl2 expression in the brains of MIA-induced mice.(A); confocal microscopic image showing immunofluorescent staining for Dpysl2 (anti-Dpysl2, red, Cy3) with NeuN in the medial prefrontal cortex. Fluorescent staining revealed a decrease of Dpysl2 in these regions. Scale bar, mPFC, 50㎛. (B); scatter gram in the graph indicates SEM (*p < 0.05 compared to CON, #,##p < 0.05 compared to MIA). mPFC; medial frontal cortex, CON; offspring of non-MIA mice, MIA; offspring of MIA mice, CLZ; offspring of MIA mice treated with clozapine drug, VF; offspring of MIA mice treated with Valeriana fauriei extract, SEM; Standard error of the mean.
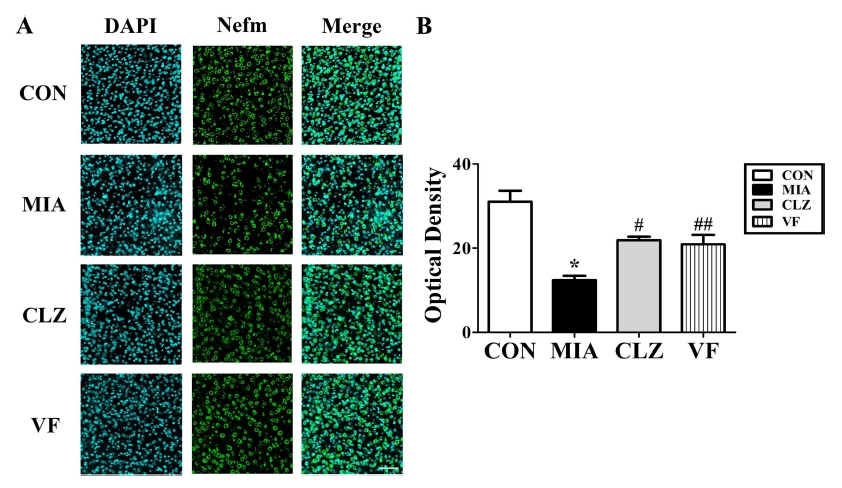
Immunohistochemical analysis of Nefm expression in the brains of MIA-induced mice.(A); confocal microscopic image showing immunofluorescent staining for Nefm (anti-Nefm, green, FITC) with DAPI (blue) in the medial prefrontal cortex. Fluorescent staining revealed a decrease of Dpysl2 in these regions. Scale bar, mPFC, 50㎛. (B); the scatter gram in the graph indicates SEM (*p < 0.05 compared to CON, #,##p < 0.05 compared to MIA). mPFC; medial frontal cortex, CON; offspring of non-MIA mice, MIA; offspring of MIA mice, CLZ; offspring of MIA mice treated with clozapine drug, VF; offspring of MIA mice treated with Valeriana fauriei extract, SEM; Standard error of the mean.
The genus Valeriana contains over 250 species including subspecies such as Valeriana fauriei Briq. (VF) and Valeriana officinalis (Circosta et al., 2007). Several previous studies have shown that VF might improve sleep quality (Bent et al., 2006). The effects of VF on spatial memory have been demonstrated using a novel object recognition and water maze test (Nam et al., 2013).
VF also has antioxidant effects and antidepressant-like activity (Liu et al., 2012). Additionally, in vitro study has shown that Valeriana officinalis extract has neuroprotective properties against Aβ toxicity (Malva et al., 2004). In this study, we proposed that VF might be able to reverse schizophrenia-like behavior in MIA offspring using an animal model.
Using an epidemiologically motivated neurodevelopmental animal model of related psychiatric disorders such as depression and schizophrenia, previous studies showed that MIA in the form of viral-like acute phase response can trigger early/middle gestation (GD9) in mice, leading to long-lasting changes in behavior and protein expression (Meyer et al., 2010b). Impaired social interaction behavior was also observed in MIA offspring. This diminution in social interaction behaviors could reflect an increase in anxiety in MIA offspring (Bitanihirwe et al., 2010). The influence of MIA is consistent with previous reports on social interaction deficits emerged in adult mice after exposing to Poly I:C in middle gestation (Smith et al., 2007). In this current study, MIA-induced increases in aggressive behavior were restored by VF treatment. In addition, some behavioral patterns in FST and OFT were recovered by the treatment.
We found PPI deficits in the offspring of MIA treated by synthetic double-stranded RNA poly I:C. Braff et al. (2001) have shown deficits in the PPI of the startle response in both schizophrenic patients and animal models of this disorder (Braff et al., 2001). Such deficits in PPI are believed to reflect disruption in sensorimotor gating. We also found that exposure to MIA disrupted the gating as reflected by deficits in PPI and the deficit may be affected by VF treatment. Clozapine antipsychotic is wellknown to be effective in treating positive and negative symptoms of schizophrenia (Manschreck et al., 1999) or reverse PPI disruption induced by MIA in offspring (Meyer et al., 2010a). The efficacy of clozapine has been attributed to its action on several different mPFC neurotransmitter systems (Johnston-Wilson et al., 2000).
In the present study, the typical antipsychotic clozapine was effective in restoring PPI deficits observed in MIA offspring treated with Poly I:C during pregnancy.
In this study, we investigated the levels of two proteins; Dpysl2 and neurofilament protein. The decrease of mPFC in neurofilament and Dpysl2 proteins due to MIA was affected by VF treatment. Dpysl2, also known as collapsin response mediator protein 2, can regulate axonal outgrowth by promoting microtubule assembly, vesicle trafficking, and synaptic physiology (Lin et al., 2011). The expression of Dpysl2 in humans has been reported to be decreased in the brains of patients with schizophrenia (Johnston-Wilson et al., 2000).
Neurofilament protein may also play a role in intracellular transport to axons and dendrites. It forms part of the axon skeleton and functionally maintains neuronal caliber (Cassereau et al., 2013). Our previous study has shown that Dpysl2 and neurofilament protein levels are decreased in stress-induced schizophrenia-like rat offspring but recovered by VF treatment (Lee et al., 2016a). These findings suggest that changes in the expression of neurodevelopmental proteins such as neurofilament proteins and Dpysl2 caused by MIA might have enduring effects on axonal outgrowth and synaptic function in MIA offspring.
Our results showed that maternal administration of Poly I:C into MIA caused abnormality of behavioral pattern, deficits in sensory-motor gating, and aggressive behavior in SIT. However, these effects could be improved by oral administration extract of VF. Some behavioral patterns in FST and OFT for the analysis of depressive behaviors were recovered by treatment with VF.
In addition, MIA-induced decrease in the expression levels of neurofilament and Dpsyl2 was restored to normal levels after treatment with VF. The present study provided valuable data regarding additional role of VF in addressing the pathogenesis of psychiatric disorders such as schizophrenia. However, further research is needed to characterize the pharmacological functions of VF using cellular and animal models.
ACKNOWLEDGEMENTS
This work was carried out with the support of Cooperative Research Program for Agriculture Science and Technology Development(PJ011582) Rural Development Administration, Republic of Korea.
References
-
Adachi, M, Barrot, M, Autry, AE, Theobald, D, Monteggia, LM, Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy, Biological Psychiatry, (2008), 63, p642-649.
[https://doi.org/10.1016/j.biopsych.2007.09.019]

-
Ahn, YS, Hur, M, An, TJ, Park, CG, Kim, YG, Park, CB, Baek, WS, Study on flowering, bearing fruit, seed harvesting and seedling transplanting cultivation ofValeriana faurieiBriquet, Korean Journal of Medicinal Crop Science, (2012), 20, p365-371.
[https://doi.org/10.7783/kjmcs.2012.20.5.365]

- Bakshi, VP, Swerdlow, NR, Geyer, MA, Clozapine antagonizes phencyclidine-induced deficits in sensorimotor gating of the startle response, Journal of Pharmacology and Experimental Therapeutics, (1994), 271, p787-794.
-
Bent, S, Padula, A, Moore, D, Patterson, M, Mehling, W, Valerian for sleep A systematic review and metaanalysis, American Journal of Medicine, (2006), 119, p1005-1012.
[https://doi.org/10.1016/j.amjmed.2006.02.026]

-
Bitanihirwe, BK, Peleg-Raibstein, D, Mouttet, F, Feldon, J, Meyer, U, Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia, Neuropsychopharmacology, (2010), 35, p2462-2478.
[https://doi.org/10.1038/npp.2010.129]

-
Braff, DL, Geyer, MA, Swerdlow, NR, Human studies of prepulse inhibition of startle Normal subjects, patient groups, and pharmacological studies, Psychopharmacology, (2001), 156, p234-258.
[https://doi.org/10.1007/s002130100810]

-
Brown, AS, Sourander, A, Hinkka-Yli-Salomki, S, McKeague, IW, Sundvall, J, Surcel, HM, Elevated maternal Creactive protein and autism in a national birth cohort, Molecular Psychiatry, (2014), 19, p259-264.
[https://doi.org/10.1038/mp.2012.197]

-
Canetta, S, Sourander, A, Surcel, HM, Hinkka-Yli-Salomki, S, Leivisk, J, Kellendonk, C, McKeague, IW, Brown, AS, Elevated maternal C-reactive protein and increased risk of schizophrenia in a national birth cohort, American Journal of Psychiatry, (2014), 171, p960-968.
[https://doi.org/10.1176/appi.ajp.2014.13121579]

-
Cassereau, J, Nicolas, G, Lonchampt, P, Pinier, M, Barthelaix, A, Eyer, J, Letournel, F, Axonal regeneration is compromised in NFH-LacZ transgenic mice but not in NFHGFP mice, Neuroscience, (2013), 228, p101-108.
[https://doi.org/10.1016/j.neuroscience.2012.10.011]

-
Chang, CH, Hsiao, YH, Chen, YW, Yu, YJ, Gean, PW, Social isolation-induced increase in NMDA receptors in the hippocampus exacerbates emotional dysregulation in mice, Hippocampus, (2015), 25, p474-485.
[https://doi.org/10.1002/hipo.22384]

-
Circosta, C, De Pasquale, R, Samperi, S, Pino, A, Occhiuto, F, Biological and analytical characterization of two extracts fromValeriana officinalis, Journal of Ethnopharmacology, (2007), 112, p361-367.
[https://doi.org/10.1016/j.jep.2007.03.021]

-
Cryan, JF, Mombereau, C, In search of a depressed mouse Utility of models for studying depression-related behavior in genetically modified mice, Molecular Psychiatry, (2004), 9, p326-357.
[https://doi.org/10.1038/sj.mp.4001457]

-
de Oliveria, DM, Barreto, G, De Andrade, DV, Saraceno, E, Aon- Bertolino, L, Capani, F, Bachá, RDSE, Giraldez, LD, Cytoprotective effect ofValeriana officinalisextract on an in vitro experimental model of Parkinson disease, Neurochemical Research, (2009), 34, p215-220.
[https://doi.org/10.1007/s11064-008-9749-y]

-
Fatemi, SH, Reutiman, TJ, Folsom, TD, Huang, H, Oishi, K, Mori, S, Smee, DF, Pearce, DA, Winter, C, Sohr, R, Juckel, G, Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring Implications for genesis of neurodevelopmental disorders, Schizophrenia Research, (2008), 99, p56-70.
[https://doi.org/10.1016/j.schres.2007.11.018]

-
Jacobo-Herrera, NJ, Vartiainen, N, Bremner, P, Gibbons, S, Koistinaho, J, Heinrich, M, NF-κB modulators fromValeriana officinalis, Phytotherapy Research, (2006), 20, p917-919.
[https://doi.org/10.1002/ptr.1972]

-
Johnston-Wilson, NL, Sims, CD, Hofmann, JP, Anderson, L, Shore, AD, Torrey, EF, Yolken, RH, Diseasespecific alterations in frontal cortex brain proteins in schizophrenia, bipolar disorder, and major depressive disorder, Molecular Psychiatry, (2000), 5, p142-149.
[https://doi.org/10.1038/sj.mp.4000696]

-
Joo, J, Lee, S, Nah, SS, Kim, YO, Kim, DS, Shim, SH, Hwangbo, Y, Kim, HK, Kwon, JT, Kim, JW, Song, HY, Kim, HJ, Lasp1 is down-regulated in NMDA receptor antagonisttreated mice and implicated in human schizophrenia susceptibility, Journal of Psychiatric Research, (2013), 47, p105-112.
[https://doi.org/10.1016/j.jpsychires.2012.09.005]

-
Kang, MG, Kim, YH, Im, AR, Nam, BS, Chae, SW, Lee, MY, Antidepressant-like effects ofSchisandra chinensisBaillon water extract on animal model induced by chronic mild stress, Korean Journal of Medicinal Crop Science, (2014), 22, p196-202.
[https://doi.org/10.7783/kjmcs.2014.22.3.196]

-
Kim, YO, Lee, HY, Won, H, Nah, SS, Lee, HY, Kim, HK, Kwon, JT, Kim, HJ, Influence of Panax ginseng on the offspring of adult rats exposed to prenatal stress, International Journal of Molecular Medicine, (2015), 35, p103-109.
[https://doi.org/10.3892/ijmm.2014.2003]

-
Lee, H, Joo, J, Nah, SS, Kim, JW, Kim, HK, Kwon, JT, Lee, HY, Kim, YO, Kim, HJ, Changes in Dpysl2 expression are associated with prenatally stressed rat offspring and susceptibility to schizophrenia in humans, International Journal of Molecular Medicine, (2015), 35, p1574-1586.
[https://doi.org/10.3892/ijmm.2015.2161]

-
Lee, H, Won, H, Im, J, Kim, YO, Lee, S, Cho, IH, Kim, HK, Kwon, JT, Kim, HJ, Effect ofValeriana faurieiextract on the offspring of adult rats exposed to prenatal stress, International Journal of Molecular Medicine, (2016), 38, p251-258.
[https://doi.org/10.3892/ijmm.2016.2589]

- Lee, YS, Lee, SW, Kim, YB, Kim, OT, Park, KH, Lee, JW, Lee, DY, Kim, GS, Kwon, DY, Han, SH, Monitoring of biological hazards in herbal crops from Korean market, Korean Journal of Medicinal Crop Science, (2016b), 24, p143-151.
-
Lin, PC, Chan, PM, Hall, C, Manser, E, Collapsin response mediator proteins(CRMPs) are a new class of microtubule-associated protein(MAP) that selectively interacts with assembled microtubules via a taxol-sensitive binding interaction, Journal of Biological Chemistry, (2011), 286, p41466-41478.
[https://doi.org/10.1074/jbc.m111.283580]

-
Liu, XG, Gao, PY, Wang, GS, Song, SJ, Li, LZ, Li, X, Yao, XS, Zhang, ZX, In vivo antidepressant activity of sesquiterpenes from the roots ofValeriana faurieiBriq, Fitoterapia, (2012), 83, p599-603.
[https://doi.org/10.1016/j.fitote.2012.01.004]

-
Malva, JO, Santos, S, Macedo, T, Neuroprotective properties ofValeriana officinalisextracts, Neurotoxicity Research, (2004), 6, p131-140.
[https://doi.org/10.1007/bf03033215]

-
Manschreck, TC, Redmond, DA, Candela, SF, Maher, BA, Effects of clozapine on psychiatric symptoms, cognition, and functional outcome in schizophrenia, The Journal of Neuropsychiatry Clinical Neurosciences, (1999), 11, p481-489.
[https://doi.org/10.1176/jnp.11.4.481]

- Meyer, U, Knuesel, I, Nyffeler, M, Feldon, J, Chronic clozapine treatment improves prenatal infection-induced working memory deficits without influencing adult hippocampal neurogenesis, Psychopharmacology, (2010a), 208, p531-543.
- Meyer, U, Spoerri, E, Yee, BK, Schwarz, MJ, Feldon, J, Evaluating early preventive antipsychotic and antidepressant drug treatment in an infection-based neurodevelopmental mouse model of schizophrenia, Schizophrenia Bulletin, (2010b), 36, p607-623.
- Miyasaka, LS, Atallah, N, Soares, B, Valerian for anxiety disorders. Cochrane Database of Systematic Reviews, (2006), John Wiley and Sons, Ltd, Chichester, Engl, 18, pCD004515, http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD004515.pub2/pdf (cited by 2016 May 20).
-
Nam, SM, Choi, JH, Yoo, DY, Kim, W, Jung, HY, Kim, JW, Kang, SY, Park, J, Kim, DW, Kim, WJ, Yoon, YS, Hwang, IK, Valeriana officinalisextract and its main component, valerenic acid, ameliorate D-galactose-induced reductions in memory, cell proliferation, and neuroblast differentiation by reducing corticosterone levels and lipid peroxidation, Experimental Gerontology, (2013), 48, p1369-1377.
[https://doi.org/10.1016/j.exger.2013.09.002]

- National Research Council(NRC), Guide for the care and use of laboratory animals, (1996), National Academy Press, Washington, DC, USA, p22.
-
Nozari, M, Shabani, M, Farhangi, AM, Mazhari, S, Atapour, N, Sex-specific restoration of MK-801-induced sensorimotor gating deficit by environmental enrichment, Neuroscience, (2015), 299, p28-34.
[https://doi.org/10.1016/j.neuroscience.2015.04.050]

-
Smith, SE, Li, J, Garbett, K, Mirnics, K, Patterson, PH, Maternal immune activation alters fetal brain development through interleukin-6, Journal of Neuroscience, (2007), 27, p10695-10702.
[https://doi.org/10.1523/jneurosci.2178-07.2007]

-
Zhu, F, Zheng, Y, Liu, Y, Zhang, X, Zhao, J, Minocycline alleviates behavioral deficits and inhibits microglial activation in the offspring of pregnant mice after administration of polyriboinosinic-polyribocytidilic acid, Psychiatry Research, (2014), 219, p680-686.
[https://doi.org/10.1016/j.psychres.2014.06.046]
