Effects of Roasting and Peeling Process and Extraction Temperature on the Antioxidant Activity of Burdock Tea
© The Korean Society of Medicinal Crop Science. All rights reserved.
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License ( http://creativecommons.org/licenses/by-nc/3.0 ) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
We investigated the optimal aqueous extraction conditions for recovery of high yields of total phenolic compounds from roots of Arctium lappa L. (burdock, Asteraceae), and we compared their antioxidant capacity.
The antioxidant activity of the extracts was tested using 2,2-diphenyl-1-picrylhydrazyl, 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)-diammonium salt, and oxygen radical absorbance capacity assays. In addition, the major phenolic compounds present in the extracts were determined by high performance liquid chromatography analysis. Our results suggest that the roasted burdock 100°C, 15 min extract exhibited the strongest radical scavenging activity and possessed the highest concentration of phenolic compounds. The polyphenol content of both dried burdock and roasted burdock significantly increased with increase in the extraction temperature and time.
These results indicated a relationship between phenolic compound levels in burdock and their free radical scavenging activities. This suggests that phenolic compounds significantly increase the antioxidant potential of burdock extracts.
Keywords:
Arctium lappa L., 2,2’-Azino-Bis (3-Ethylbenzothiazoline-6 Sulfonic Acid)-Diammonium Salt, Antioxidant Activity, 2,2-Diphenyl-1-Picrylhydrazyl, Oxygen Radical Absorbance CapacityINTRODUCTION
Considerable epidemiological evidence has revealed an association between diets rich in vegetables and fruits and a decreased risk of cardiovascular diseases and certain forms of cancer (Gaté et al., 1999; Yeum et al., 2003). Research has shown that, these diseases occur result from a variety of exogenous and endogenous free radicals in the external environment.
The most widely used natural antioxidants are carotenoids, vitamin C, and vitamin E. These antioxidants play a significant role in reducing excessive oxidative stresses, which leads to loss of cell function and regulation (Nordberg and Arnr, 2001). Excessive levels of reactive oxygen species (ROS) or loss of antioxidant defense systems can injure cells through lipid peroxidation, and cause DNA and protein damage (Halliwell, 1996; Shahidi et al., 1992). Therefore, improving antioxidant potential is an important method to prevent the development of some chronic diseases.
Herb- and plant-based medicines contain phenolic compounds and have played an important role in human health. Accumulating evidence suggests that phenolic compounds function as reducing agents, hydrogen donors, and free- radical eliminators (Lee et al., 2011; Seo et al., 2009; Shahidi et al., 1992). However, a lot of research is still needed to evaluate their properties and mechanisms of action.
Arctium lappa L. commonly known as burdock, is a perennial plant of the Asteraceae (Compositae) family. Burdock root is usually consumed as a tea and a side dish in Korea. It has been reported to have a variety of biological activities including anti-inflammatory, antiproliferative, antiviral, and antioxidant effects (da Silva et al., 2013; dos Santos et al., 2008; Huang et al., 2010; JianFeng et al., 2012; Pereira et al., 2005; Wang et al., 2014). Previous studies have focused on the antioxidant activity of organic solvent extracts of burdock root; however, it is unknown whether water extracts of burdock root possess antioxidant properties (da Silva et al., 2013; dos Santos et al., 2008; Tian et al., 2014). Because burdock tea is prepared from water, it is necessary to assay the antioxidant activity of its water extract. During the preparation of burdock tea, it is dried and roasted without being peeled in the food industry; however, the skin is often peeled following a preliminary wash in the home. Therefore, the antioxidant activity of non-peeled and peeled burdock root should be studied.
The purpose of this study was to optimize water extraction conditions for high yield total phenolic content recovery. The antioxidant capacity of water extracts of burdock root was assessed and compared using three different antioxidant assays. Additionally, the contents of major phenolic compounds in burdock root extracts were determined by high performance liquid chromatography (HPLC) analysis. These findings can be considered for the further isolation and purification of phenolic compounds from A. lappa root for potential use in the nutraceutical or pharmaceutical industries.
MATERIALS AND METHODS
1. Sample preparation
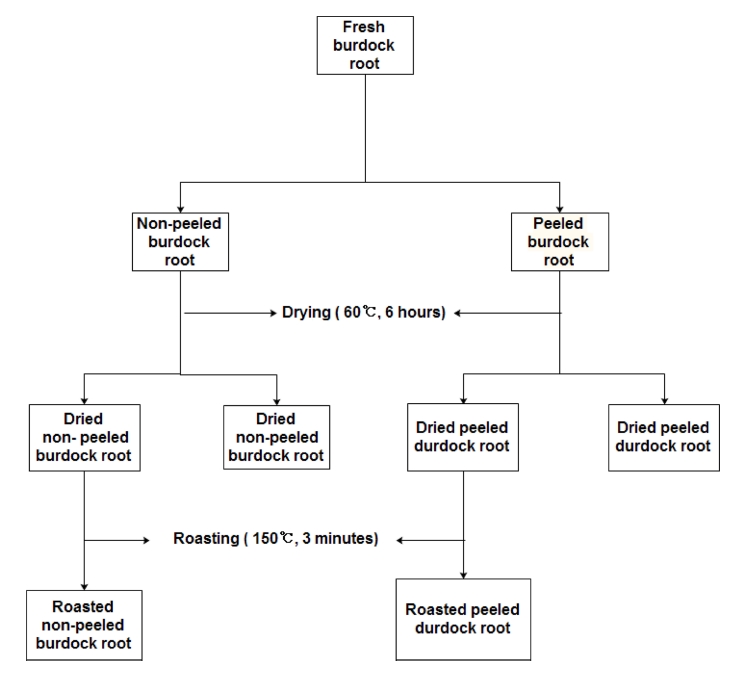
The roots of dried- and roasted-burdock were obtained from Jinjusi, Gyeongsangnam-do, South Korea, and fresh roots were obtained from Asan-si, Chungcheongnam-do, South Korea. The fresh burdock roots were divided into two groups. One group contained peeled burdock roots and the other contained non-peeled burdock roots. The burdock roots were cut into thin slices (0.5㎝), and then dried for 6 h at 60℃ using a drying oven (J-300M, JISICO Co., Ltd., Seoul, Korea). After drying, the two groups of burdock were divided into further two subgroups, one of which was roasted separately in a fryingpan for 3 min at 150℃ (Fig. 1). Water was selected for this study as it is safe, environmentally friendly, accessible, and cheap in comparison to the organic solvents utilized in previous studies (Vuong et al., 2014).
The roots of dried burdock (DB) and roasted burdock (RB) (1 g) were extracted with water (20, 80, 100℃, 100㎖) for 5, 15, and 30 min. Furthermore, the nonpeeled and peeled burdock roots (1 g) were extracted with 80℃ water (100㎖) for 30 min, next, all the burdock extracts were filtered through 0.45㎛ FP-Vericel™ membrane filter (VWR International, LLC., Radnor, PA, USA). All other chemicals and reagents used were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
2. Total phenol determination
Total phenol contents were determined using the Folin- Ciocalteu reagent according to the method described by Ainsworth and Gillespie (2007). Briefly, 50㎕ samples of various fractions were assayed with 250㎕ Folin reagent and 500㎕ sodium carbonate (20%, w/v). The mixture was vortexed and diluted with water to a final volume of 5㎖. After incubation for 30 min at room temperature, the absorbance was read at 734㎚ using UV-VIS spectrophotometer (V-530, Jasco Co., Tokyo, Japan). Total polyphenol contents of burdock extracts were reported as ㎎• gallic acid equivalent/g (㎎• GAE/g).
3. Total flavonoid determination
Total flavonoid contents were determined using the method described by Ahmed et al. (2014). Burdock extracts (10㎎/㎖) were added to the test tube, then 2.5㎖ distilled water and 75㎕ 5% NaNO2 solution were added, and the samples were shaken. After 5min, 150㎕ 10% AlCl3 solution was added, and after 6min, 500㎕ 1N NaOH solution was added and the mixture was shaken. After incubation for 10min at 37℃, the absorbance was determined at 415㎚ using UV-VIS spectrophotometer (V- 530, Jasco Co., Tokyo, Japan). Total flavonoid contents of burdock extracts were expressed as ㎎• quercetin equivalent/g (㎎• QE/g).
4. DPPH radical scavenging activity
The antioxidant activity of the extract and fractions was determined on the basis of their ability to scavenge the stable 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical, using the method described by Braca et al. (2001). The absorbance at 517㎚ was determined by using UV-VIS spectrophotometer (V-530, Jasco Co., Tokyo, Japan). The DPPH radical scavenging activity was calculated by the following formula:
where A0 is the absorbance of the control and A is the absorbance of the burdock extracts or the standard.
5. ABTS radical scavenging activity
The spectrophotomeric analysis of 2,2'-azino-bis (3- ethylbenzothiazoline-6-sulfonic acid)-diammonium salt (ABTS) radical scavenging activity of burdock extracts was performed according to the method described by Re et al. (1999). The absorbance at 734㎚ was determined by using UV-VIS spectrophotometer (V-530, Jasco Co., Tokyo, Japan). The ABTS•+ radical scavenging activity was calculated using the following equation:
where Asample is the absorbance of the burdock extracts or the standard and Acontrol is the absorbance of the control.
6. ORAC value assay
The oxygen radical absorbance capacity (ORAC) value was determined using the modified method described by Ou et al. (2001). The reaction was carried out in 75mM sodium phosphate buffer (KH2PO4-K2HPO4 buffer, pH 7.4, 25㎕) and fluorescein (60㎚, 150㎕) with burdock extracts (100㎍/㎖, 25㎕), 2,2’-azobis (2-methylpropionamidine) dihydrochloride (AAPH, 25㎕) and trolox (25㎕), respectively. Samples were mixed was done in black-walled 96-well plates at 37℃, and testing started immediately after mixing. Fluorescence was measured at an excitation wavelength of 485㎚ and emission wavelength 535㎚ every 1min for 120min using a microplate reader Fluostar Omega spectrophotometer (BMG Labtech, Ortenberg, Germany).
The trolox calibration curve was calculated based on the trolox concentration range of 0 - 50㎍/㎖ (1, 5, 10, 30, and 50㎍/㎖ final concentrations). The area under the curve (AUC) was calculated for each sample by integrating the relative fluorescence curve. The net AUC of the sample was calculated by subtracting the AUC of the blank. The regression equation between the AUC and the trolox concentration was determined, and ORAC values are expressed as ㎎• trolox equivalent/100 g (㎎• TE/100 g) of samples.
7. Quantification of phenolic compounds by HPLC analysis
Phenolic compounds were identified and quantified by using a HPLC Spectra System SCM 1000 (Thermo Fisher Scientific Inc., Waltham, MA, USA) equipped with UVvisible detector (Shimadzu, Kyoto, Japan). Reverse phase chromatographic analyses were carried out under gradient conditions using a packed C18 column (4.6㎜ × 120㎜); the mobile phase was water containing 0.5% phosphoric acid and 20% ACN. The burdock extracts as well as the mobile phase were filtered through a 0.45㎛ membrane filter (Merck Millipore, Billerica, MA, USA) and then degassed in an ultrasonic bath prior to use. The extracts were analyzed for the presence or absence of gallic acid (GA), chlorogenic acid (CGA), epigallocatechin gallate (EGCG), and caffeic acid (CA). Those compounds were identified by comparing their retention time and UV absorption spectra with those of the commercial standards. The flow rate was 0.5㎖/min, injection volume was 10㎕ and the wavelength was 280㎚.
8. Statistical analysis
All tests were carried out in triplicate and the data are presented as means ± standard deviation (SD). Data were analyzed by One-way analysis of variance (ANOVA) followed by Duncan’s Multiple Range Tests; p < 0.05 was considered as statistically significant.
RESULTS
1. Total phenolic and flavonoid contents of DB and RB extracts
Plant phenolics and flovonoids are generally highly effective free radical scavengers and antioxidants (Mustafa et al., 2010). The content of total phenolic compounds in the extracts from DB and RB are shown in Table 1. The total polyphenol contents of DB and RB ranged from 10.86 ± 0.33 to 38.75 ± 0.48, and 27.20 ± 0.42 to 83.97 ± 2.02㎎• GAE/g, respectively. The total flavonoid contents of DB and RB ranged from 2.17 ± 0.09 to 25.33 ± 0.41 and from 4.68 ± 0.41 to 33.32 ± 0.25, ㎎• QE/g, respectively (Table 1). Longer extraction time and higher extract temperature resulted in the extraction of higher phenolic and flavonoid compounds. In the comparison between the DB and RB extracts, using the same extraction method, the RB extracts showed higher total polyphenol contents and total flavonoid contents than the DB extracts.
In Table 2, the total phenolic and flavonoid contents of the non-peeled RB extracts were higher than those of the other extracts (p < 0.05).
2. DPPH radical scavenging activity of burdock extracts
The free-radical scavenging activity of burdock extracts was assessed by DPPH assay. As shown in Fig. 2A, the RB 100℃, 30 min extract showed the highest scavenging activity. The RB extracts showed higher DPPH radical scavenging activity than DB extracts with the same extraction method.
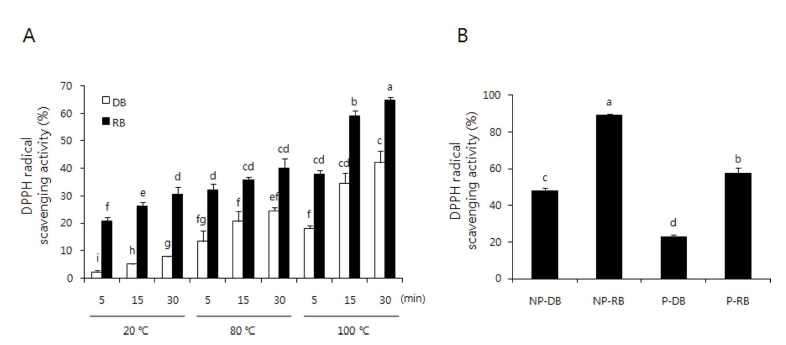
DPPH radical scavenging activity of DB, RB, NP-B and P-B extract.A; black or white bar represents roasted-burdock (RB) or dried-burdock (DB), respectively. B; non peeled-dried burdock (NP-DB), non peeled-roasted burdock (NP-RB), peeled-dried burdock (P-DB), peeled-roasted burdock (P-RB). Each bar represents the mean ± SD. The bars with different letters are significantly different (p < 0.05) from each other.
The DPPH radical scavenging activity of non-peeled and peeled burdock extracts decreased in the order non-peeled RB (88.98 ± 0.65%) > peeled RB (57.53 ± 2.74%) > non-peeled DB (47.82 ± 1.50%) > peeled DB (22.89 ± 0.81%). The RB extracts showed higher scavenging activity than DB extracts, and non-peeled burdock extracts showed higher scavenging activity than peeled burdock extracts (Fig. 2B).
3. ABTS radical scavenging activity of burdock extracts
The ABTS assay is based on the reaction between ABTS and potassium persulfate, which generates a blue/ green ABTS radical (ABTS•+). The ABTS radical scavenging activities of various extracts increased with extract time and temperature (Fig. 3A). The ABTS radical scavenging activity of non-peeled and peeled burdock extracts decreased in the order non-peeled RB (30.43 ± 0.48%) > peeled RB (17.45 ± 0.41%) > non-peeled DB (12.99 ± 0.34%) > peeled DB (10.27 ± 0.44%). Similar to the results obtained by the DPPH radical scavenging assay, the RB extracts showed higher scavenging activity than DB extracts, and nonpeeled burdock extracts showed higher ABTS radical scavenging activity than peeled burdock extracts (Fig. 3B).
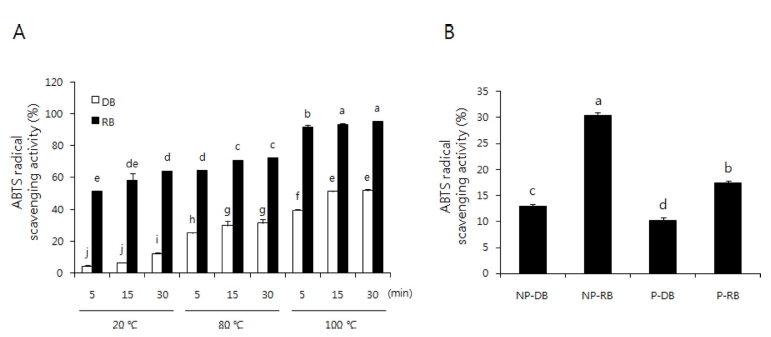
ABTS radical scavenging activity of DB, RB, NP-B and P-B extract.A; black or white bar represents roasted-burdock (RB) or dried-burdock (DB), respectively. B; non peeled-dried burdock (NP-DB), non peeled-roasted burdock (NP-RB), peeled-dried burdock (P-DB), peeled-roasted burdock (P-RB). Each bar represents the mean ± SD. The bars with different letters are significantly different (p < 0.05) from each other.
4. ORAC value of burdock extracts
The ORAC assay is based on radical scavenging by the AAPH radical, and the ORAC has been widely employed to evaluate the antioxidant capacity of beverages, food, and plant extracts (Prior et al., 2005). Similar to the DPPH and ABTS scavenging activity, obtained in the same extract time, the ORAC value increased as the temperature increased, and the RB extracts showed higher values than the DB extracts (Fig. 4A). Non-peeled burdock extracts showed higher ORAC values than peeled burdock extracts (Fig. 4B). The ORAC values of nonpeeled and peeled burdock extracts decreased in the order non-peeled RB (7993.29 ± 74.90㎎• TE/100 g) > non-peeled DB (3306.03 ± 41.72㎎• TE/100 g) > peeled RB (2322.49 ± 76.94㎎• TE/100 g) > peeled DB (1,081.85 ± 40.90㎎• TE/ 100 g). These data indicate that the presence of phenolic and flavonoid compounds in extracts contributes significantly to their antioxidative potential.
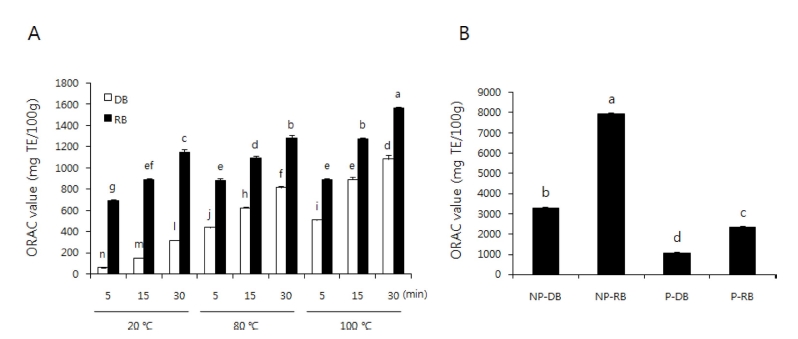
ORAC value of DB, RB, NP-B and P-B extract.A; black or white bar represents roastedburdock (RB) or dried-burdock (DB), respectively. B; non peeled-dried burdock (NP-DB), non peeled-roasted burdock (NP-RB), peeled-dried burdock (P-DB), peeled-roasted burdock (P-RB). Each bar represents the mean ± SD. The bars with different letters are significantly different (p < 0.05) from each other.
5. Quantification of phenolic compounds by HPLC analysis
Previous studies have identified GA, CGA, and CA in burdock roots (Tezuka et al., 2013; Saleem et al., 2009). As the extract temperature and time were increased, the polyphenol contents of both DB and RB significantly increased (Table 3).
DISCUSSION
Burdock (Arctium lappa L.) root has been widely used as a folk medicine and vegetable in East Asia. In the present study, we investigated the optimal water extraction conditions for high-yield total phenolic content recovery, and the antioxidant capacity of the water extracts of A. lappa root.
In the DPPH radical scavenging assay, ABTS radical scavenging assay, and ORAC assay, the RB 100℃, 30 min extract showed the highest antioxidant activity, and non-peeled burdock extracts showed higher scavenging activity than peeled burdock extracts (Fig. 2, 3, 4). The ABTS radical scavenging activity of RB (80 - 100℃) is almost two-fold higher than that of DB (Fig. 3). As expected, the total phenolic acid and flavonoid contents of the RB 100℃, 30 min sample were higher than those of the other extracts (Table 1). These results indicate that there is a relationship between the phenolic compound concentrations in burdock and their free radical scavenging activities. Therefore, the presence of phenolic compounds significantly contributes to their antioxidant potential. These data are consistent with those from previous studies showing that a highly positive relationship exists between total phenolic contents and antioxidant activity in many plants (Chukwumah et al., 2009; Gursoy et al., 2009).
The phenolic and flavonoids compound contents of nonpeeled burdock extracts are greater than those of peeled burdock extracts (Table 2). These results support the conclusions of others studies showing that fruits and vegetable peels possess a higher content of these compounds than pulps (Asikin et al., 2012; Gorinstein et al., 2001).
Although caffeoylquinic acids, dicaffeoylquinic acids, CA derivatives, hydroxycinnamoylquinic acids, and CGA have been identified in burdock roots (Jaiswal and Kuhnert, 2011; Lin and Harnly, 2008; Liu et al., 2012; Saleem et al., 2009), most studies analyzed the organic solvent extract of burdock roots. Since burdock root has been consumed as a tea, we analyzed phenolic compounds in the water extract of burdock roots. As the extract temperature and time increased, the contents of phenolic compounds also increased (Table 3).
The CGA content was highest among the three phenolic compounds in DB (Table 3). The GA content increased as much as the CGA content in RB. The RB 100℃, 30 min extract contained 10-fold more CGA than the DB 100℃, 30 min extract (Table 3). Roasting treatment led to a significant increase in the level of GA, CGA, and CA, which correlates well with the observed antioxidant activity of burdock root extract. GA is a strong antioxidant compound and is used as a reference in the assessment of antioxidant activity. Its oxidation involves 4.6 electrons, whereas ascorbic acid involves two electrons (Hotta et al., 2002). CGA is the ester of CA and quinic acid and also possesses antioxidant properties (Sato et al., 2011; Xu et al., 2012). During the roasting process, GA may be released from tannin and CA may be generated from CGA degradation.
Thus, the antioxidant activity of the RB 100℃, 30 min extract is partly due to the presence of those phenolic compounds. The results indicate that the roasting process and conditions play an important role in the phenolic compound content of burdock tea. In addition to burdock root phenolic compounds, water-soluble polysaccharide, fructo-oligosaccharide, arctigenin and inulin are also known to have antioxidant activity (Li et al., 2008; Liu et al., 2014; Zhao et al., 2009). The antioxidant activity of burdock root may involve a synergistic effect among phytochemicals.
In this study, an assay of antioxidant activity was tested for the first time and the total phenolic and flavonoid contents of water extracts from burdock root were determined. Antioxidant and radical scavenging ability of the extracts were found to be extract temperature and time dependent.
A key finding of this study was that the RB 100℃, 30 min extract exhibited the strongest radical scavenging activity and possessed the highest concentration of phenolic compounds. Unfortunately, we did not analyze the contents of various phenolic compounds or those in non-peeled and peeled burdock extracts. However, the non-peeled RB extract was found to have a higher ORAC value than peeled RB extract, suggesting that non-peeled burdock extract has more antioxidant benefits than peeled burdock extract. Roasting treatment generated a high amount of phenolic compounds, in particular, GA.
Thus, non-peeled RB can be very beneficial for enhanced phenolic compound extraction with desirable benefits. Future studies should focus on the roasting condition, the identification of these antioxidants, and the purification of this plant ingredient to obtain agents with high efficacy and activity.
ACKNOWLEDGEMENT
This research was supported by the Soonchunhyang University Research Fund.
References
-
Ahmed, D, Fatima, K, Saeed, R, Analysis of phenolic and flavonoid contents, and the anti-oxidative potential and lipid peroxidation inhibitory activity of methanolic extract of Carissa opaca roots and its fractions in different solvents, Antioxidants, (2014), 3, p671-683.
[https://doi.org/10.3390/antiox3040671]

-
Ainsworth, EA, Gillespie, KM, Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent, Nature Protocols, (2007), 2, p875-877.
[https://doi.org/10.1038/nprot.2007.102]

-
Asikin, Y, Taira, I, Inafuku, S, Sumi, H, Sawamura, M, Takara, K, Wada, K, Volatile aroma components and antioxidant activities of the flavedo peel extract of unripe Shiikuwasha(Citrus depressaHayata), Journal of Food Science, (2012), 77, pC469-C475.
[https://doi.org/10.1111/j.1750-3841.2011.02604.x]

- Braca, A, de Tommasi, N, di Bari, L, Pizza, C, Politi, M, Morelli, I, Antioxidant principles from Bauhinia tarapotensis, Journal of Natural Products, (2001), 64, p892-895.
-
Chukwumah, Y, Walker, LT, Verghese, M, Peanut skin color A biomarker for total polyphenolic content and antioxidative capacities of peanut cultivars, International Journal of Molecular Sciences, (2009), 10, p4941-4952.
[https://doi.org/10.3390/ijms10114941]

-
da Silva, LM, Allemand, A, Mendes, DAGB, dos Santos, AC,=, André, E, de Souza, Lm, Cipriani, TR, Dartora, N, Marques, MCA, Baggio, CH, Werner, MF, Ethanolic extract of roots fromArctium lappaL. accelerates the healing of acetic acid-induced gastric ulcer in rats: Involvement of the antioxidant system, Food and Chemical Toxicology, (2013), 51, p179-187.
[https://doi.org/10.1016/j.fct.2012.09.026]

-
dos Santos, AC, Baggio, CH, Freitas, CS, Lepieszynski, J, Mayer, B, Twardowschy, A, Missau, FC, dos Santos, EP, Pizzolatti, MG, Marques, MC, Gastroprotective activity of the chloroform extract of the roots fromArctium lappa, L, Journal of Pharmacy and Pharmacology, (2008), 60, p795-801.
[https://doi.org/10.1211/jpp.60.6.0016]

- Gaté, L, Paul, J, Ba, GN, Tew, KD, Tapiero, H, Oxidative stress induced in pathologies The role of antioxidants, Biomedicine and Pharmacotherapy, (1999), 53, p169-180.
-
Gorinstein, S, Zachwieja, Z, Folta, M, Barton, H, Piotrowicz, J, Zemser, M, Weisz, M, Trakhtenberg, S, Màrtín-Belloso, O, Comparative contents of dietary fiber, total phenolics, and minerals in persimmons and apples, Journal of Agricultural and Food Chemistry, (2001), 49, p952-957.
[https://doi.org/10.1021/jf000947k]

-
Gursoy, N, Sihoglu-Tepe, A, Tepe, B, Determination ofIn vitroantioxidative and antimicrobial properties and total phenolic contents of Ziziphora clinopodioides, Cyclotrichium niveum, and Mentha longifolia ssp. typhoides var. typhoides, Journal of Medicinal Food, (2009), 12, p684-689.
[https://doi.org/10.1089/jmf.2008.0102]

-
Halliwell, B, Antioxidants in human health and disease, Annual Review of Nutrition, (1996), 16, p33-50.
[https://doi.org/10.1146/annurev.nutr.16.1.33]

-
Hotta, H, Nagano, S, Ueda, M, Tsujino, Y, Koyama, J, Osakai, T, Higher radical scavenging activities of polyphenolic antioxidants can be ascribed to chemical reactions following their oxidation, Biochimica et Biophysica Acta, (2002), 1572, p123-132.
[https://doi.org/10.1016/s0304-4165(02)00285-4]

-
Huang, TC, Tsai, SS, Liu, LF, Liu, YL, Liu, HJ, Chuang, KP, Effect ofArctium lappaL. in the dextran sulfate sodium colitis mouse model, World Journal of Gastroenterology, (2010), 16, p4193-4199.
[https://doi.org/10.3748/wjg.v16.i33.4193]

-
Jaiswal, R, Kuhnert, N, Identification and characterization of five new classes of chlorogenic acids in burdock(Arctium lappaL) roots by liquid chromatography/tandem mass spectrometry, Food and Function, (2011), 2, p63-71.
[https://doi.org/10.1039/c0fo00125b]

- JianFeng, C, PengYing, Z, ChengWei, X, TaoTao, H, YunGui, B, KaoShan, C, Effect of aqueous extract ofArctium lappaL.(burdock) roots on the sexual behavior of male rats, BMC Complementary and Alternative Medicine, (2012), 12, p8-8.
-
Lee, JH, Park, AR, Choi, DW, Kim, JD, Kim, JC, Ahn, JH, Lee, HY, Choe, M, Choi, KP, Shin, IC, Park, HJ, Analysis of chemical compositions and electron-donating ability of 4 Korean wild Sannamuls, Korean Journal of Medicinal Crop Science, (2011), 19, p111-116.
[https://doi.org/10.7783/kjmcs.2011.19.2.111]

-
Li, D, Kim, JM, Jin, Z, Zhou, J, Prebiotic effectiveness of inulin extracted from edible burdock, Anaerobe, (2008), 14, p29-34.
[https://doi.org/10.1016/j.anaerobe.2007.10.002]

-
Lin, LZ, Harnly, JM, Identification of hydroxycinnam oylquinic acids of arnica flowers and burdock roots using a standardized LC-DAD-ESI/MS profiling method, Journal of Agricultural and Food Chemistry, (2008), 56, p10105-10114.
[https://doi.org/10.1021/jf802412m]

-
Liu, J, Cai, YZ, Wong, RNS, Lee, CKF, Tang, SCW, Sze, SCW, Tong, Y, Zhang, Y, Comparative analysis of caffeoylquinic acids and lignans in roots and seeds among various burdock(Arctium lappa) genotypes with high antioxidant activity, Journal of Agricultural and Food Chemistry, (2012), 60, p4067-4075.
[https://doi.org/10.1021/jf2050697]

- Liu, W, Wang, J, Zhang, Z, Xu, J, Xie, Z, Slavin, M, Gao, X, In vitroand in vivo antioxidant activity of a fructan from the roots ofArctium lappaL, International Journal of Biological Macromolecules, (2014), 65, p446-453.
-
Mustafa, RA, Abdul Hamid, A, Mohamed, S, Bakar, FA, Total phenolic compounds, flavonoids, and radical scavenging activity of 21 selected tropical plants, Journal of Food Science, (2010), 75, pC28-C35.
[https://doi.org/10.1111/j.1750-3841.2009.01401.x]

- Nordberg, J, Arnr, ES, Reactive oxygen species, antioxidants, and the mammalian thioredoxin system, Free Radical Biology and Medicine, (2001), 31, p1287-1312.
-
Ou, B, Hampsch-Woodill, M, Prior, RL, Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe, Journal of Agricultural and Food Chemistry, (2001), 49, p4619-4626.
[https://doi.org/10.1021/jf010586o]

-
Pereira, JV, Bergamo, DCB, Pereira, JO, Frana, SDC, Pietro, RCLR, Silva-Sousa, YTC, Antimicrobial activity ofArctium lappaconstituents against microorganisms commonly found in endodontic infections, Brazilian Dental Journal, (2005), 16, p192-196.
[https://doi.org/10.1590/s0103-64402005000300004]

-
Prior, RL, Wu, X, Schaich, K, Standardized methods for the determination of antioxidant capacity and phenolic in foods and dietary supplements, Journal of Agricultural and Food Chemistry, (2005), 53, p4290-4302.
[https://doi.org/10.1021/jf0502698]

-
Re, R, Pellegrini, N, Proteggente, A, Pannala, A, Yang, M, Rice- Evans, C, Antioxidant activity applying an improved ABTS radical cation decolorization assay, Free Radical Biology and Medicine, (1999), 26, p1231-1237.
[https://doi.org/10.1016/s0891-5849(98)00315-3]

-
Saleem, A, Walshe-Roussel, B, Harris, C, Asim, M, Tamayo, C, Sit, S, Arnason, JT, Characterisation of phenolics in Flor-Essence®: A compound herbal product and its contributingherbs, Phytochemical Analysis, (2009), 20, p395-401.
[https://doi.org/10.1002/pca.1139]

-
Sato, Y, Itagaki, S, Kurokawa, T, Ogura, J, Kobayashi, M, Hirano, T, Sugawara, M, Iseki, K, In vitroand in vivo antioxidant properties of chlorogenic acid and caffeic acid, International Journal of Pharmaceutics, (2011), 403, p136-138.
[https://doi.org/10.1016/j.ijpharm.2010.09.035]

- Seo, JS, Lee, TH, Lee, SM, Lee, SE, Seong, NS, Kim, J, Inhibitory effects of methanolic extracts of medicinal plants on nitric oxide production in activated macrophage RAW 264.7 cells, Korean Journal of Medicinal Crop Science, (2009), 17, p173-178.
- Shahidi, F, Janitha, PK, Wanasundara, PD, Phenolic antioxidants, Critical Reviews in Food Science and Nutrition, (1992), 32, p67-103.
-
Tezuka, Y, Yamamoto, K, Awale, S, Lia, F, Yomoda, S, Kadota, S, Anti-austeric activity of phenolic constituents of seeds ofArctium lappa, Natural Product Communications, (2013), 8, p463-466.
[https://doi.org/10.1177/1934578x1300800414]

-
Tian, X, Sui, S, Huang, J, Bai, JP, Ren, TS, Zhao, QC, Neuroprotective effects ofArctium lappaL. roots against glutamate-induced oxidative stress by inhibiting phosphorylation of p38, JNK and ERK 1/2 MAPKs in PC12 cells, Environmental Toxicology and Pharmacology, (2014), 38, p189-198.
[https://doi.org/10.1016/j.etap.2014.05.017]

-
Vuong, QV, Goldsmith, CD, Dang, TT, Nguyen, VT, Bhuyan, DJ, Sadeqzadeh, E, Scarlett, CJ, Bowyer, MC, Optimisation of ultrasound-assisted extraction conditions for phenolic content and antioxidant capacity fromEuphorbia tirucalliusing response surface methodology, Antioxidants, (2014), 3, p604-617.
[https://doi.org/10.3390/antiox3030604]

-
Wang, P, Wang, B, Chung, S, Wu, Y, Henning, SM, Vadgama, JV, Increased chemopreventive effect by combining arctigenin, green tea polyphenol and curcumin in prostate and breast cancer cells, RSC Advances, (2014), 4, p35242-35250.
[https://doi.org/10.1039/c4ra06616b]

-
Xu, JG, Hu, QP, Liu, Y, Antioxidant and DNAprotectiveactivities of chlorogenic acid isomers, Journal of Agricultural and Food Chemistry, (2012), 60, p11625-11630.
[https://doi.org/10.1021/jf303771s]

-
Yeum, KJ, Aldini, G, Chung, HY, Krinsky, NI, Russell, RM, The activities of antioxidant nutrients in human plasma depend on the localization of attacking radical species, The Journal of Nutrition, (2003), 133, p2688-2691.
[https://doi.org/10.1093/jn/133.8.2688]

- Zhao, F, Wang, L, Liu, K, In vitroanti-inflammatory effects of arctigenin, a lignan fromArctium lappaL., through inhibition on iNOS pathway, Journal of Ethnopharmacology, (2009), 122, p457-462.