Therapeutic Potential of Chinese Prescription Hachimi-Jio-Gan and Its Crude Drug Corni Fructus against Diabetic Nephropathy
© The Korean Society of Medical Crop Science. All rights reserved
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License ( http://creativecommons.org/licenses/by-nc/3.0 ) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Traditional plant drugs, are less toxic and free from side effects compared to general synthetic drugs. They have been used for the treatment of diabetes and associated renal damage. In this study, we evaluated effect of Hachimi-jio-gan against diabetic renal damage in a rat model of type 1 diabetic nephropathy induced by subtotal nephrectomy plus streptozotocin (STZ) injection, and in Otsuka Long-Evans Tokushima Fatty (OLETF) rats and db/db mice as a model of human type 2 diabetes, and its associated complications. To explore the active components of Hachimi-jio-gan, the antidiabetic effect of corni fructus, a consituent of Hachimi-jio-gan, and 7-O-galloyl-D-sedoheptulose, a phenolic compound isolated from corni fructus, were investigated.
We conducted an extensive literature search, and all required data were collected and systematically organized. The findings were reviewed and categorized based on relevance to the topic. A summary of all the therapeutic effects were reported as figures and tables.
Hachimi-jio-gan serves as a potential therapeutic agent to against the development of type 1 and type 2 diabetic nephropathy. From the results of characterization active components of corni fructus, 7-O-galloyl-D-sedoheptulose is considered to play an important role in preventing and/or delaying the onset of diabetic renal damage. 7-O-Galloyl-D-sedoheptulose is expected to serve as a novel therapeutic agent against the development of diabetic nephropathy.
Keywords:
Corni Fructus, Diabetic Nephropathy, Hachimi-Jio-Gan, 7-O-Galloyl-D-SedoheptuloseINTRODUCTION
Diabetic nephropathy is one of the major diabetic microvascular complications, along with retinopathy and neuropathy, and it is characterized by albuminuria, hypertension, a decline of the glomerular filtration rate (GFR), and glomerular sclerosis (Remuzzi and Bertani, 1998). During its development, glucose exerts toxic actions as a result of processes that are activated within the diabetic kidney, that is, the accumulation of advanced glycation endproducts (AGEs), increase in oxidative stress, abnormal polyol metabolism, and synthesis of growth factors (Lehmann and Schleicher, 2000).
For two decades, clinical and experimental studies have provided evidence of various beneficial effects of glycemic control, angiotensin II receptor blockers, angiotensinconverting enzyme inhibitors, and antihypertensive drugs against diabetic nephropathy (UKPDS, 1998; HOPE, 2000; Brenner et al., 2001; Lewis et al., 2001). However, large numbers of patients in many countries are still suffering from diabetic nephropathy.
Traditionally, in Japan, China, and Korea, some Kampo prescriptions that are usually derived from natural sources have long been used for the improvement of subjective symptoms of diabetes and its complications. In particular, Hachimi-jio-gan ameliorates hyperglycemia, so it is used clinically to improve several disorders associated with diabetes (Goto et al., 1989; Furuya et al., 1999), and it has been used widely for the treatment of renal dysfunction in human subjects (Yamada, 1992). Furthermore, Hachimijio- gan has long been used widely to treat several chronic diseases, including chronic nephritis, sterility, and vegetative ataxia (Huang, 1997), although scientific evidence supporting a pharmacological basis for its therapeutic effects has yet to be published. Previously, we performed in vitro and in vivo studies using twelve Chinese prescriptions to investigate the possibility of curing diabetic nephropathy (Yokozawa et al., 2001a), and demonstrated the effects of the oral administration of four Kampo prescriptions: Ompi-to (Wen-Pi-Tang), Keishi-bukuryo-gan (Gui-Zhi-Fu-Ling-Wan), Sairei-to (Chai-Ling-Tang), and Hachimi-jio-gan (Ba-Wei-Di-Huang-Wan), in an animal model of diabetic nephropathy by measuring biochemical parameters that are affected by persistent hyperglycemia (Nakagawa et al., 2001). Among these prescriptions, Hachimi-jio-gan is expected to provide a novel therapeutic approach against diabetic nephropathy.
Therefore, we evaluated the effects of Hachimi-jio-gan and identified its active compound, ameliorating diabetic renal damage using streptozotocin (STZ)-induced type 1 diabetes, a type 1 diabetic nephropathy rat model which underwent a subtotal nephrectomy plus STZ injection, and Otsuka Long-Evans Tokushima Fatty (OLETF) rats and db/db mice as a model of human type 2 diabetes and its complications.
MATERIALS AND METHODS
1 Hachimi-jio-gan
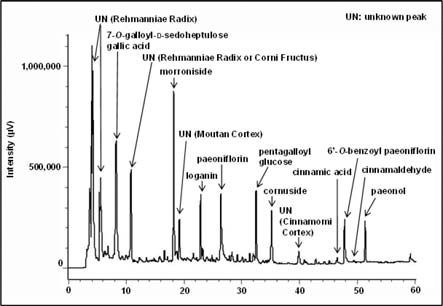
The composition of Hachimi-jio-gan was as follows: rehmanniae radix (Rehmannia glutinosa Libosch. Var. purpurea Makino) 6 g, corni fructus (Cornus officinalis Sieb. et Zucc.) 3 g, dioscoreae rhizoma (Dioscorea japonica Thunb.) 3 g, alismatis rhizoma (Alisma orientale Juzep.) 3 g, hoelen (Poria cocos Wolf) 3 g, moutan cortex (Paeonia suffruticosa Andrews) 2.5 g, cinnamomi cortex (Cinnamomum cassia Blume) 1.0 g, and aconiti tuber (Aconitum carmichaeli Debx.) 0.5 g. The above-mentioned crude drugs were boiled together gently in ten times their volume of water for 60 min, filtered, and the filtrate was spray-dried to obtain the extract at a yield of about 10%, by weight, of the original preparation. For the analysis of the components of Hachimi-jio-gan, HPLC chromatography was performed. As shown in Fig. 1, 7-O-Galloyl-Dsedoheptulose, gallic acid, morroniside, loganin, paeoniflorin, pentagalloyl glucose, cornuside, cinnamic acid, 6’-O-benzoyl paeoniflorin, cinnamaldehyde, and paeonol were detected as the major components of Hachimi-jio-gan.
2 Effect of Hachimi-jio-gan on type 1 diabetic nephropathy
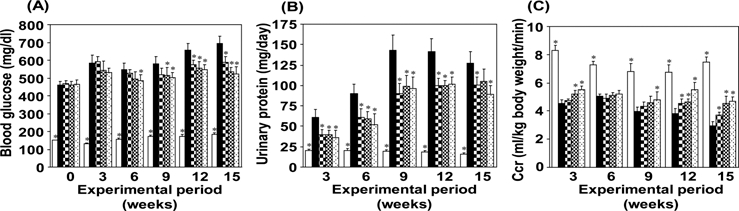
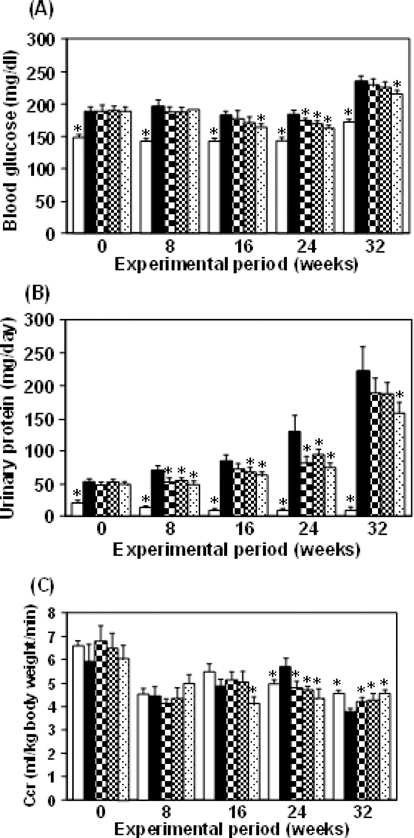
Diabetic nephropathy is characterized as advanced kidney disease caused by longitudinal hyperglycemia and its metabolic abnormalities. Thus, this study involved longterm administration in order to show the effect of Hachimi-jio-gan on advanced kidney disease in diabetic nephropathy. The rats receiving sub-total nephrectomy and STZ injection showed metabolic abnormalities and renal lesions resembling diabetic nephropathy in humans (Yokozawa et al., 2001b). During the experimental period, the serum glucose and urinary protein excretion levels were remarkably higher in the rat model employed in this study than in normal rats (Fig. 2A, B), indicating that disorders of glucose metabolism and changes in the capillary filtration barrier result in the increased permeability of the glomerular basement membrane. In addition, this rat model showed a significant decrease in creatinine clearance (CCr) (Fig. 2C), which is an effective index for expressing the GFR (Bell, 1991). However, the present investigation demonstrated that the administration of Hachimi-jio-gan reduced the serum glucose and urinary protein excretion levels, but increased CCr (Fig. 2A-C), suggesting that the effective control of glucose metabolism is a therapeutic target for preventing diabetic complications including diabetic nephropathy. Moreover, the decreased serum albumin level in this animal model was reversed by the administration of Hachimi-jio-gan. On the basis of these results, it was found that STZ injection to subtotally nephrectomized rats resulted in progressive diabetic nephropathy, and Hachimi-jio-gan prevented or delayed it (Yokozawa et al., 2004a).
HPLC profile of Hachimi-jio-gan extract.Blood glucose (A), urinary protein (B) and CCr (C) in normal rats (□) and in diabetic nephropathy rats treated with either Hachimi-jio-gan. 50㎎/㎏ body weight, 100㎎/㎏ body weight/day, 200㎎/㎏ body weight/day or control (■) for 15 weeks. Data are the means ± SEM. *p < 0.05 vs. control rats with diabetic nephropathy. Figure was taken from Yokozawa et al., 2001bet al. (2004a).
Diabetic nephropathy is also well-known as a chronic disease which causes a non-enzymatic glycation reaction, an abnormal polyol pathway, and increases oxidative stress, which all synergistically affect renal mesangial cells, progressing glomerulosclerosis through some steps (Brownlee et al., 1984; Cooper et al., 1998; Lehmann and Schleicher, 2000). The glycosylated serum protein level increased in the animal model we used, which suggests that sugar oxidation was stimulated, increasing damage to both sugars and proteins in the circulation and vascular walls, continuing and reinforcing the cycle of oxidative stress and damage. In addition, the accumulation of AGEs in the kidney was also observed. However, Hachimi-jio-gan decreased the levels of glycosylated serum proteins and AGEs significantly and dose-dependently, suggesting that it can inhibit oxidative and irreversible renal damage caused by protein glycation reactions. Also, our study showed that renal sorbitol levels were markedly elevated in rats with diabetic nephropathy compared with normal rats, but the administration of Hachimi-jio-gan significantly decreased the sorbitol level, suggesting that the disturbance of the glucose-dependent metabolic pathway and irreversible tissue damage caused by such disturbance under conditions of diabetic nephropathy would be ameliorated by decreasing the activity of the polyol pathway and inhibiting the protein glycation reaction. Moreover, the serum and renal thiobarbituric acid (TBA)-reactive substance levels were measured to determine the effects of Hachimi-jio-gan on oxidative stress in relation to the development of diabetic nephropathy. Lipid peroxidation levels in the serum and kidney were significantly elevated in rats with diabetic nephropathy compared with normal rats, while the administration of Hachimi-jio-gan reduced these levels. These findings suggest that the administration of Hachimijio- gan would ameliorate the oxidative stress associated with diabetic nephropathy through the inhibition of lipid peroxidation and, thus, it would result in the improvement of renal lesions caused by oxidative stress (Yokozawa et al., 2004a).
The clinical manifestations of diabetic nephropathy, notably proteinuria, hypertension, and renal insufficiency, are closely associated with the severity of renal lesions (Winetz et al., 1982; Adler, 1997). In the animal model used, the histopathological features of diabetic nephropathy, glomerular sclerosis, and tubulointerstitial lesions were observed. A substantial body of evidence from cell culture experiments and experimental models of diabetic nephropathy suggests that progressive renal damage is the ultimate expression of the pathological consequences of accumulating abnormalities of the glomerulus and tubulointerstitium (Winetz et al., 1982; Yaqoob et al., 1994; Adler, 1997). Hachimi-jio-gan had a significant protective effect on the renal lesions, as demonstrated by the histopathological evaluations, and Fig. 3 shows typical photomicrographs of kidney tissue obtained from each group, suggesting that Hachimi-jio-gan improves the renal dysfunction associated with renal lesions. Therefore, the results of the present study confirm that Hachimi-jio-gan has a protective effect in diabetic nephropathy rats through the amelioration of metabolic disorders, oxidative stress, and renal dysfunction associated with renal lesions (Yokozawa et al., 2004a).
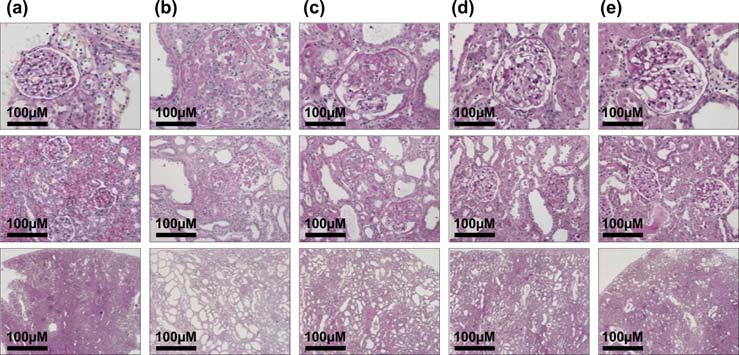
Photomicrographs of the glomeruli (upper panel, × 200), tubulus (middle panel, × 100) and interstitium (lower panel, × 20) obtained from normal rats (a), diabetic nephropathy rats in the control (b) and Hachimi-jio-gan-treated [50㎎/㎏ body weight/day (c), 100㎎/㎏ body weight/day (d) and 200㎎/㎏ body weight/day (e)] groups. Figure was taken from Yokozawa et al. (2004a).
3 Effect of Hachimi-jio-gan on type 2 diabetic nephropathy
OLETF rats were used as a model of human type 2 diabetes (Kawano et al., 1992), and Hachimi-jio-gan was orally administered for 32 weeks. Male OLETF diabetic rats compared with Long-Evans Tokushima Otsuka (LETO) control rats over the time course maintained a higher body weight as well as food and water consumption levels, but Hachimi-jio-gan-treated groups did not show any differences, while Hachimi-jio-gan reduced the increase of the serum glucose level in OLETF diabetic rats from the latter half of the administration period (Fig. 4A). On the other hand, OLETF control rats showed marked proteinuria, but the oral administration of Hachimi-jio-gan markedly reduced the urinary protein excretion rates from an early stage and this was maintained up to the end of the experimental period (Fig. 4B). In addition, Ccr levels were higher in untreated OLETF rats than LETO or Hachimi-jio-gan-treated OLETF rats at 24 weeks of administration, indicating glomerular hyperfiltration, which led to a decline in their renal function at 32 weeks. However, the abnormal renal function was normalized by the administration of Hachimi-jio-gan (Fig. 4C), suggesting that long-term treatment with Hachimi-jio-gan may counteract renal damage and delay end-stage renal disease (Yamabe and Yokozawa, 2006.
Blood glucose (A), urinary protein (B) and CCr (C) in LETO rats (□) and in OLETF rats treated with either Hachimi-jio-gan. 50㎎/㎏ body weight, 100㎎/㎏ body weight/day, 200㎎/㎏ body weight/day or control (■) for 32 weeks. Data are the means ± SEM. *p < 0.05 vs. untreated OLETF rats. Figure was taken from Yamabe and Yokozawa (2006).
We clarified that Hachimi-jio-gan ameliorated the oxidative and irreversible renal damage caused by protein glycation reactions using a type 1 diabetic nephropathy rat model (Yokozawa et al., 2004a). A number of mechanisms contribute to the development of kidney damage in diabetes, that is, glucotoxicity, oxidative stress, AGE accumulation, fibrogenesis, and cytokine production such as transforming growth factor-β (TGF-β), which may be influenced by activation of the islet rennin-angiotensin system (Fukami et al., 2004). OLETF rats showed significantly elevated serum glycosylated protein and renal AGE levels, but Hachimi-jio-gan treatment significantly and dose-dependently reduced them to below the levels of non-diabetic LETO rats. In addition, we investigated the TBA-reactive substance levels in the serum and kidney to clarify the influence of Hachimi-jio-gan on oxidative stress. The long-term administration of Hachimi-jio-gan reduced renal TBA-reactive substance levels significantly, despite only showing a tendency to reduce serum levels without significance. These findings suggest that the effect of Hachimi-jio-gan in reducing oxidative stress caused by the binding activity of AGEs, plasma proteins, and receptors for AGEs (RAGE) in glomerular mesangial cells or macrophages is superior to its effect against markers of oxidative stress in the serum. Furthermore, Hachimi-jio-gan suppressed not only TGF-β1 and fibronectin protein synthesis but also nuclear factor-kappa B (NF-κB)-induced immune and inflammatory factors, inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (Cox-2) (Yamabe and Yokozawa, 2006). Taken together, Hachimi-jio-gan may ameliorate functional abnormalities in association with the angiotensin II-TGF-β signaling pathway in mesangial cells induced by AGE-RAGE-mediated reactive oxygen species, which cause fibronectin overexpression in mesangial cells, leading to glomerular sclerosis.
4 Which is the main contributor among the eight crude drugs in Hachimi-jio-gan?
There have been many experiments focusing on the treatment of diabetes and its complications with herbal medicines including traditional Chinese prescriptions. According to our previous study, it was discovered that Hachimi-jio-gan had effects on metabolic disorders, especially on AGE formation and elevated oxidative stress in diabetic nephropathy (Nakagawa et al., 2001), while we have demonstrated that Keishi-bukuryo-gan showed potential therapeutic effects on diabetic nephropathy via reducing oxidative stress (Nakagawa et al., 2003). In addition, we also clarified that the administration of dried rehmanniae radix extract, which is the main constituent of Hachimi-jio-gan, attenuates renal dysfunction in diabetic nephropathy mainly due to its suppression of oxidative stress (Yokozawa et al., 2001bet al., 2004b); However, for the analysis of this prescription, further characterization of the other constituents is needed. According to the HPLC profile, 7-Ogalloyl- D-sedoheptulose, gallic acid, morroniside, loganin, paeoniflorin, pentagalloyl glucose, cornuside, cinnamic acid, 6’- O-benzoyl paeoniflorin, cinnamaldehyde, and paeonol were detected as the major components of Hachimi-jio-gan (Fig. 1). 7-O-Galloyl-D-sedoheptulose, morroniside, and loganin are components of corni fructus (Wang et al., 2003; Park et al., 2012), and paeoniflorin is the component of moutan cortex common in Keishi-bukuryo-gan (Kaneda et al., 1972). Therefore, on the assumption that corni fructus is a major contributor to the effect of Hachimi-jio-gan, the following study was carried out.
5 Corni fructus
Corni fructus has been used as a traditional medicine in China, Japan, and Korea. It has been reported that corni fructus has several biological activities, including hypoglycemic, antineoplastic, and antimicrobial effects, and to improve liver and kidney functions (Chang et al., 2004; Liou et al., 2004; Vareed et al., 2006). We previously reported that treatment with corni fructus ameliorated hyperglycemia, proteinuria, renal AGE formation, and related protein expressions, i.e., RAGE, NF- κB, TGF-β1, and Nε-(carboxymethyl)lysine (CML), in the same way as aminoguanidine. However, improvement of the renal function, shown via serum Cr and CCr, was superior to that with aminoguanidine treatment (Yamabe et al., 2007). In addition, the administration of corni fructus inhibited the elevation of both systolic and diastolic blood pressures, and lowered serum total cholesterol levels with a decrease in esterified cholesterol in the diet-induced hypercholesterolemia rat model (Park et al., 2009). Moreover, the atherogenic index was decreased in a dosedependent manner, suggesting its protective role against cardiovascular disease through regulating cholesterol and lipoprotein levels (Park et al., 2009). Thus, corni fructus may be helpful to prevent and/or delay the onset of diabetes and diabetic complications.
6 Efficacy-based identification of active component of corni fructus
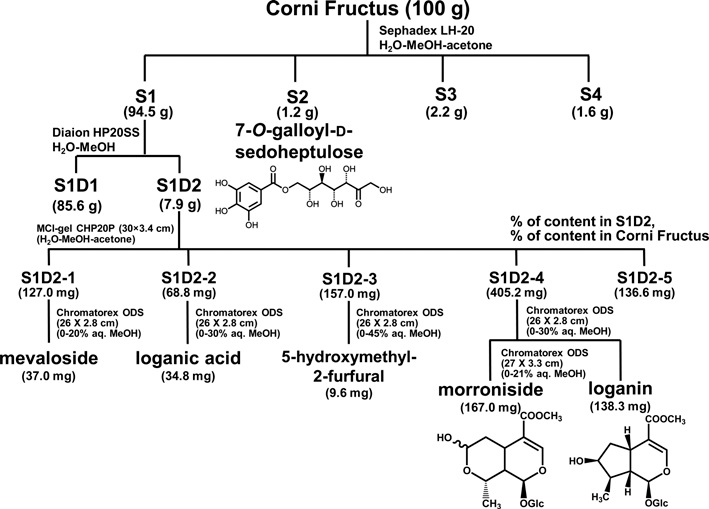
The discovery of efficacious components is essential for clarification of the precise mechanisms of herbal medicines. However, studies on the biological activities of the active components in corni fructus are very poorly. Hence, we have isolated the active components of corni fructus by employing activity-guided fractionation (Fig. 5). The major components of morroniside, loganin, or 7-Ogalloyl- D-sedoheptulose are considered to be a beneficial effect on diabetes and diabetic complications. 7-O-Galloyl-Dsedoheptulose is only detected as a compound from corni fructus, and its biological activity has not known until now except for our previous research. For these reasons, we further clarified the mechanisms of 7-O-Galloyl-Dsedoheptulose acting against diabetic kidney disease in expectation of identifying it as the novel active contributor in corni fructus.
7 7-O-Galloyl-D-sedoheptulose
To investigate the effect of 7-O-Galloyl-D-sedoheptulose, db/db mice were used. Being a spontaneous mutant strain, the C57BLKS/J db/db mouse, has the db mutation, a splicing mutation caused by a point mutation in the downstream intron of the leptin receptor gene, and so it is unresponsive to leptin. Leptin is a peptide hormone secreted by adipocytes and is involved in eating behavior and energy homeostasis. For this reason, after birth, homozygous diabetic (db/db) mice show unrepressed eating behavior, become obese, and develop severe insulin resistance associated with hyperinsulinemia and hyperglycemia (Sharma et al., 2003). In the present study, db/db mice showed the symptoms of diabetes, such as hyperglycemia, hyperleptinemia, and hyperinsulinemia, compared with homozygous control (m/m) mice, as shown in Table 1.
7-O-Galloyl-D-sedoheptulose treatment significantly decreased serum leptin and insulin levels at a dose of 100㎎/㎏, while the serum glucose level was slightly decreased without significance. The serum C-peptide level was compared as an indirect biomarker of insulin secretion. As expected, there was a significant increase in the serum Cpeptide level in the vehicle-treated db/db group, which was closely associated with the increased removal of blood glucose (Park et al., 2013). Thus, 7-O-Galloyl-Dsedoheptulose administration prevents diabetes in db/db mice, as evidenced by improved insulin sensitivity through the maintenance of normal insulin and glucose levels and the preservation of insulin and C-peptide levels in the serum, meaning that 7-O-Galloyl-D-sedoheptulose can ameliorate impaired glucose and insulin tolerance in db/db mice.
Diabetic renal damage is known to involve hyperglycemia-induced oxidative stress. Increased oxygen and peroxy radicals worsen tissue oxidative stress, which affects the oxidation of important macromolecules including proteins, lipids, carbohydrates, and DNA chains. Moreover, reactive oxygen species (ROS) activate the signal transduction cascade and transcription factors and overexpression of genes and proteins in glomerular mesangial and tubular epithelial cells, leading to pathological changes in the kidney (Lee et al., 2003). Therefore, we investigated the effect of 7-O-Galloyl-D-sedoheptulose on the oxidative stress and ROS-related factors involved in the development of diabetes-induced renal damage using db/db mice. As shown in Fig. 6, 7-O-Galloyl-D-sedoheptulose effectively attenuated oxidative stress via a decrease in ROS and TBA-reactive substance levels as well as an enhanced reduced glutathione (GSH)/oxidized glutathione (GSSG) ratio. In addition, increased serum urea nitrogen and creatinine levels associated with an abnormal renal function were significantly lowered by 7-O-Galloyl-D-sedoheptulose treatment (Table 2) (Park et al., 2012).
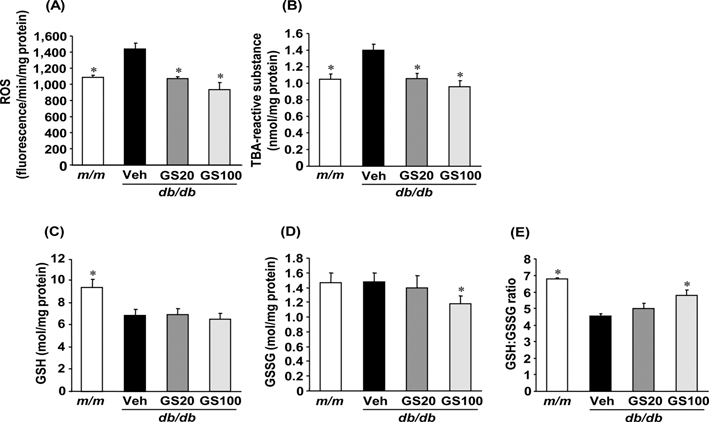
ROS (A), TBA-reactive substance (B), GSH (C), GSSG (D) and GSH/GSSG (E) levels in the kidney of type 2 diabetic db/db mice treated with 7-O-Galloyl-Dsedoheptulose for 8 weeks. m/m; misty, Veh; vehicle-treated db/db mice, GS20; 7- O-galloyl-D-sedoheptulose 20㎎/㎏ body weight-treated db/db mice, GS100; 7-Ogalloyl- D-sedoheptulose 100㎎/㎏ body weight-treated db/db mice. Data are the means ± SEM. *p < 0.05 vs. vehicle-treated db/db mouse values. Figure was taken from Park et al., (2012).
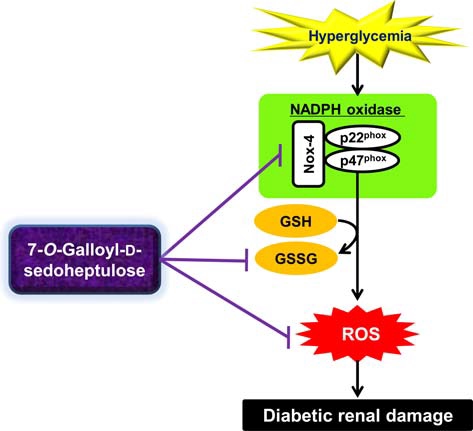
Although the origin of increased ROS generation in renal disease is multifactorial, recent studies have focused on the fact that nicotinamide adenine dinucleotide phosphate (NADPH) oxidase mainly participates in the process of ROS generation (Geiszt et al., 2000; Nath and Norby, 2000; Shiose et al., 2001). Renal NADPH oxidase expression was reported to be enhanced in glomeruli and distal tubules in the presence of diabetic nephropathy (Gill and Wilcox, 2006). Structurally, NADPH oxidase comprises the membrane-associated cytochrome b558, composed of one p22phox and one gp91phox subunit and at least four cytosolic subunits (p47phox, p67phox, p40phox, and the small GTPase rac1 or rac2) (Babior et al., 2002). In a rodent model of type 2 diabetes (db/db mouse), the renal expression of Nox-4 and p22phox was increased, and this was associated with ROS-induced renal damage (Sedeek et al., 2010). Hence, we examined the renal protein expression of Nox-4 and p22phox, subunits of NADPH oxidase, to identify the exact mechanism behind the reduction of renal ROS levels in the 7-O-Galloyl-D-sedoheptulose-treated group. In western blot analysis, Nox-4 and p22phox protein expressions were significantly upregulated in the type 2 diabetic kidney; however, 7-O-Galloyl-D-sedoheptulose administration at 100㎎/㎏ significantly normalized the increased subunits of NADPH oxidase (Fig. 7) (Park et al., 2012). These results indicate that the inhibitory effect of 7-O-Galloyl-Dsedoheptulose on ROS generation was due to the downregulated expression of NADPH oxidase in db/db mice.
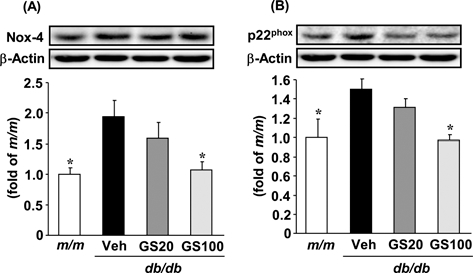
Nox-4 (A) and p22phox (B) protein expressions in the kidney of type 2 diabetic db/db mice treated with 7-O-Galloyl-D-sedoheptulose for 8 weeks. m/ m; misty, Veh;vehicle-treated db/db mice, GS20; 7-Ogalloyl- D-sedoheptulose 20㎎/㎏ body weight-treated db/db mice, GS100; 7-O-Galloyl-D-sedoheptulose 100㎎/㎏ body weight-treated db/db mice. Data are the means ± SEM. *p < 0.05 vs. vehicle-treated db/ db mouse values. Figure was taken from Park et al. (2012).
This study supports the concept that, in hyperglycemia, enhanced oxidative stress and the upregulation of NADPH oxidase are associated with renal damage in type 2 diabetes. 7-O-Galloyl-D-sedoheptulose administration effectively alleviated these unfavorable responses in the presence of diabetic injury of the kidney, as shown in Fig. 8. Therefore, the present study can accumulate the knowledge on the beneficial effects of bioactive constituents of corni fructus, as well as the possible development of therapeutic or preventive agents for diabetic complications.
RESULTS AND DISCUSSION
We have studied Hachimi-jio-gan in order to establish a therapeutic strategy for the prevention of diabetic nephropathy using rat models. These results indicate that Hachimi-jio-gan could have a protective role either in early or advanced stages of type 1 and 2 diabetic nephropathy via the amelioration of several metabolic disorders caused by hyperglycemia and also a renoprotective effect. To add to these findings, we clarified that corni fructus (Cornus officinalis Sieb. et Zucc.) has similar effects to Hachimijio- gan, and also successfully identified the most important contributor to the effect of Hachimi-jio-gan, i.e., 7-Ogalloyl- D-sedoheptulose, which was isolated from corni fructus. This component is expected to become a novel therapeutic agent against the development of diabetic nephropathy.
REFERENCES
- Adler, S, Structure-function relationships in diabetic nephropathy Lessons and limitations, Kidney International, (1997), 60, pS42-S45.
- Babior, BM, Lambeth, JD, Nauseef, W, The neutrophil NADPH oxidase, Archives of Biochemistry and Biophysics, (2002), 397, p342-344.
-
Bell, DSH, Diabetic nephropathy Changing concepts of pathogenesis and treatment, The American Journal of the Medical Sciences, (1991), 301, p195-200.
[https://doi.org/10.1097/00000441-199103000-00009]

-
Brenner, BM, Cooper, ME, de Zeeuw, D, Keane, WF, Mitch, WE, Parving, HH, Remuzzi, G, Snapinn, SM, Zhang, Z, Shahinfar, S, Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy, The New England Journal of Medicine, (2001), 345, p861-869.
[https://doi.org/10.1056/nejmoa011161]

-
Brownlee, M, Vlassara, H, Cerami, A, Nonenzymatic glycosylation and the pathogenesis of diabetic complications, Annals of Internal Medicine, (1984), 101, p527-537.
[https://doi.org/10.7326/0003-4819-101-4-527]

-
Chang, JS, Chiang, LC, Hsu, FF, Lin, CC, Chemoprevention against hepatocellular carcinoma of Cornus officinalis in vitro, The American Journal of Chinese Medicine, (2004), 32, p717-725.
[https://doi.org/10.1142/s0192415x04002296]

- Cooper, ME, Gilbert, RE, Epstein, M, Pathophysiology of diabetic nephropathy, Metabolism, (1998), 47, p3-6.
-
Fukami, K, Ueda, S, Yamagishi, S, Kato, S, Inagaki, Y, Takeuchi, M, Motomiya, Y, Bucala, R, Iida, S, Tamaki, K, Imaizumi, T, Cooper, ME, Okuda, S, AGEs activate mesangial TGF-?-Smad signaling via an angiotensin II type I receptor interaction, Kidney International, (2004), 66, p2137-2147.
[https://doi.org/10.1111/j.1523-1755.2004.66004.x]

- Furuya, Y, Kawakita, T, Tajima, S, Effect of Hachimijio- gan(Ba-Wei-Di-Huang-Wan) on insulin resistance in noninsulin dependent diabetes mellitus model mice, Journal of Traditional Medicines, (1999), 16, p123-128.
-
Geiszt, M, Kopp, JB, Várnai, P, Leto, TL, Identification of redox, an NAD(P)H oxidase in kidney, Proceedings of the National Academy of Sciences of the United States of America, (2000), 97, p8010-8014.
[https://doi.org/10.1073/pnas.130135897]

-
Gill, PS, Wilcox, CS, NADPH oxidases in the kidney, Antioxidants and Redox Signaling, (2006), 8, p1597-1607.
[https://doi.org/10.1089/ars.2006.8.1597]

-
Goto, M, Inoue, H, Seyama, Y, Yamashita, S, Inoue, O, Yumioka, E, Comparative effects of traditional Chinesemedicines(Dai-saiko-to, Hatimi-zio-gan and Byakko-ka-ninzin-to) on experimental diabetes and hyperlipidemia, Nihon Yakurigaku Zasshi, (1989), 93, p179-186.
[https://doi.org/10.1254/fpj.93.179]

- Heart Outcomes Prevention Evaluation(HOPE), Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: Results of the HOPE study and MICRO-HOPE substudy, The Lancet, (2000), 355, p253-259.
- Huang, T, A handbook of traditional Chinese prescriptions of dubious and complicated cases, (1997), China Medical and Pharmaceutical Science and Technology Publishing House, Beijing, China, p527-538.
- Kaneda, M, Iitaka, Y, Shibata, S, Chemical studies on the oriental plant drugs-XXXIII The absolute structures of paeoniflorin, albiflorin, oxypaeoniflorin and benzoylpaeoniflorin isolated from chinese paeony root, Tetrahedron, (1972), 28, p4309-4317.
- Kawano, K, Hirashima, T, Mori, S, Saitoh, Y, Kurosumi, M, Natori, T, Spontaneous long-term hyperglycemic ratwith diabetic complications Otsuka Long-Evans Tokushima Fatty(OLETF) strain, Diabetes, (1992), 41, p1422-1428.
-
Lee, HB, Yu, MR, Yang, Y, Jiang, Z, Ha, H, Reactive oxygen species-regulated signaling pathways in diabetic nephropathy, Journal of the American Society of Nephrology, (2003), 14, pS241-S245.
[https://doi.org/10.1097/01.asn.0000077410.66390.0f]

-
Lehmann, R, Schleicher, ED, Molecular mechanism of diabetic nephropathy, Clinica Chimica Acta, (2000), 297, p135-144.
[https://doi.org/10.1016/s0009-8981(00)00240-0]

-
Lewis, EJ, Hunsicker, LG, Clarke, WR, Berl, T, Pohl, MA, Lewis, JB, Ritz, E, Atkins, RC, Rohde, R, Raz, I, Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes, The New England Journal of Medicine, (2001), 345, p851-860.
[https://doi.org/10.1056/nejmoa011303]

-
Liou, SS, Liu, IM, Hsu, SF, Cheng, JT, Corni fructus as the major herb of Die-huang-wan for lowering plasma glucose in Wistar rats, Journal of Pharmacy and Pharmacology, (2004), 56, p1443-1447.
[https://doi.org/10.1211/0022357044670]

- Nakagawa, T, Yokozawa, T, Terasawa, K, A study of Kampo medicines in a diabetic nephropathy model, Journal of Traditional Medicines, (2001), 18, p161-168.
-
Nakagawa, T, Yokozawa, T, Terasawa, K, Nakanishi, K, Therapeutic usefulness of Keishi-bukuryo-gan for diabeticnephropathy, Journal of Pharmacy and Pharmacology, (2003), 55, p219-227.
[https://doi.org/10.1211/002235702450]

-
Nath, KA, Norby, SM, Reactive oxygen species and acute renal failure, The American Journal of Medicine, (2000), 109, p665-678.
[https://doi.org/10.1016/s0002-9343(00)00612-4]

-
Park, CH, Cho, EJ, Yokozawa, T, Protection against hypercholesterolemia by corni fructus extract and its related protective mechanism, Journal of Medicinal Food, (2009), 12, p973-981.
[https://doi.org/10.1089/jmf.2009.0037]

-
Park, CH, Noh, JS, Tanaka, T, Yokozawa, T, 7-OGalloyl- D-sedoheptulose ameliorates renal damage triggered by reactive oxygen species-sensitive pathway of inflammation and apoptosis, Journal of Pharmacy and Pharmacology, (2012), 64, p1730-1740.
[https://doi.org/10.1111/j.2042-7158.2012.01559.x]

- Park, CH, Noh, JS, Park, JC, Yokozawa, T, Beneficial effect of 7-O-galloyl-D-sedoheptulose, a polyphenol isolated from corni fructus, against diabetes-induced alterations inkidney and adipose tissue of type 2 dianetic db/db mice, (2013), Egypt Cairo, Hindawi Publishing Corporation, 2013:736856. https://www.hindawi.com/journals/ecam/2013/736856/ (cited by 2013 Nov 20) 2013, p736856.
-
Remuzzi, G, Bertani, T, Pathophysiology of progressive nephropathies, The New England Journal of Medicine, (1998), 339, p1448-1456.
[https://doi.org/10.1056/nejm199811123392007]

-
Sedeek, M, Callera, G, Montezano, A, Gutsol, A, Heitz, F, Szyndralewiez, C, Page, P, Kennedy, CRJ, Burns, KD, Touyz, RM, Hébert, RL, Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney Implications in type 2 diabetic nephropathy, American Journal of Physiology-Renal Physiology, (2010), 299, pF1348-F1358.
[https://doi.org/10.1152/ajprenal.00028.2010]

- Sharma, K, McCue, P, Dunn, SR, Diabetic kidney disease in the db/db mouse, American Journal of Physiology- Renal Physiology, (2003), 284, pF1138-F1144.
-
Shiose, A, Kuroda, J, Tsuruya, K, Hirai, M, Hirakata, H, Naito, S, Hattori, M, Sakaki, Y, Sumimoto, H, A novel uperoxide-producing NAD(P)H oxidase in kidney, Journal of Biological Chemistry, (2001), 276, p1417-1423.
[https://doi.org/10.1074/jbc.m007597200]

- UK Prospective Diabetes Study(UKPDS), Intensive blood-glucose control with sulphonylurea or insulin compared with conventional treatment and risk of complications inpatients with type 2 diabetes(UKPDS 33), The Lancet, (1998), 352, p837-853.
-
Vareed, SK, Reddy, MK, Schutzki, RE, Nair, MG, Anthocyanins in Cornus alternifolia, Cornus controversa, Cornus kousa and Cornus florida fruits with health benefits, Life Sciences, (2006), 78, p777-784.
[https://doi.org/10.1016/j.lfs.2005.05.094]

-
Wang, SF, Chen, XG, Hu, ZD, Ju, Y, Analysis of three effective components in fructus corni and its preparations by micellar electrokinetic capillary chromatography, Biomedical Chromatography, (2003), 17, p306-311.
[https://doi.org/10.1002/bmc.247]

-
Winetz, JA, Golbetz, HV, Spencer, RJ, Lee, JA, Myers, BD, Glomerular function in advanced human diabetic nephropathy, Kidney International, (1982), 21, p750-756.
[https://doi.org/10.1038/ki.1982.93]

-
Yamabe, N, Yokozawa, T, Activity of the Chinese prescription Hachimi-jio-gan against renal damage in the Otsuka Long-Evans Tokushima Fatty rat A model of human type 2 diabetes mellitus, Journal of Pharmacy and Pharmacology, (2006), 58, p535-545.
[https://doi.org/10.1211/jpp.58.4.0014]

-
Yamabe, N, Kang, KS, Goto, E, Tanaka, T, Yokozawa, T, Beneficial effect of corni fructus, a constituent of Hachimi-jio-gan, on advanced glycation end-product-mediatedrenal injury in streptozotocin-treated diabetic rats, Biological and Pharmaceutical Bulletin, (2007), 30, p520-526.
[https://doi.org/10.1248/bpb.30.520]

- Yamada, T, Kinki Yoryaku, (1992), Tokyo Japan, Kyouwa-Kikaku, p1-7.
- Yaqoob, M, McClelland, P, Patrick, AW, Stevenson, A, Mason, H, White, MC, Bell, GM, Evidence of oxidant injury and tubular damage in early diabetic nephropathy, QJM An International Journal of Medicine, (1994), 87, p601-607.
- Yokozawa, T, Nakagawa, T, Terasawa, K, Effects of oriental medicines on the production of advanced glycation end products, Journal of Traditional Medicines, (2001a), 18, p107-112.
- Yokozawa, T, Nakagawa, T, Wakaki, K, Koizumi, F, Animal model of diabetic nephropathy, Experimental and Toxicologic Pathology, (2001b), 53, p359-363.
- Yokozawa, T, Yamabe, N, Cho, EJ, Nakagawa, T, Oowada, S, A study on the effects to diabetic nephropathy of Hachimi-jio-gan in rats, Nephron Experimental Nephrology, (2004a), 97, pe38-e48.
- Yokozawa, T, Kim, HY, Yamabe, N, Amelioration of diabetic nephropathy by dried rehmanniae radix(Di-huang) extract, The American Journal of Chinese Medicine, (2004b), 32, p829-839.