Antimutagenic Effect of Mulberry Leaf Extract
© The Korean Society of Medicinal Crop Science. All rights reserved.
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License ( http://creativecommons.org/licenses/by-nc/3.0 ) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The present study was carried out to asses whether mulberry leaves (MLs) have the potential to inhibit the mutagenic effect of cigarette smoke condensates (CSCs).
ML powder was extracted with 70% ethanol, and a yield of 35.1% by weight was obtained. The 70% ethanol extract of ML was further extracted sequentially using diethyl ether, chloroform, butanol, dichloromethane and water. The crude 70% ethanol extract of MLs and its solvent fractions did not show any mutagenic effect when tested at concentrations up to 1 ㎎/plate against Salmonella typhimurium TA98. In contrast, the crude 70% ethanol extract showed an inhibitory activity against the mutagenicity of CSCs in the presence of S-9 mixture. Among the solvent fractions, the diethyl ether fraction showed the highest inhibitory activity, which increased in a dose-dependent manner, inhibiting mutagenesis by approximately 97.1% at a concentration of 1㎎/plate.
In this study, we found that a crude 70% ethanol extract of MLs and the diethyl ether fraction themselves are potentially not mutagenic, but inhibit the mutagenic effect of CSCs.
Keywords:
Morus alba L., Salmonella typhimurium TA98, Antimutagenicity, Ames Test, Leaf Extract, SafetyINTRODUCTION
Recently, many studies have reported on possible cures for various cancers or to be able to suppress cancer metastasis (Han et al., 2016; Greenwell and Rahman, 2015). Natural products are composed of primary metabolites and secondary metabolites of biosynthesis in plants. Primary metabolites are unique to plants and are common to all plant sources: however, secondary metabolites exist only in specific plants, are small in quantity, and have multiple physiological activities. Many studies have also reported that plant constituents induce mutagenicity in secondary metabolites (Brusick, 2008) or inhibit mutations caused by various mutagens, thereby having a protective effect in accordance with mutation theory (Hong and Lyu, 2012).
Three species of mulberry trees are native to Korea- Morus alba L., Morus Bombycis, and Morus multicaulis from which the leaf, berry, stalk, and root have all been exploited for medicinal purposes (Choi et al., 2016). Mulberry (Morus alba L.) has been used pharmaceutically for attenuating the duration of bronchitis and acute pneumonia with good effect, for reducing swelling because of its diuretic activity, and for lowering high blood pressure. Since ancient times, mulberry has been known to be useful for treating hemoptysis, neuralgia, muscle paralysis, hemiplegia, and pruritus (Lee et al., 2011). Mulberry has also been reported to attenuate osteoarthrosis (Khunakornvichaya et al., 2016), prevent arteriosclerosis (Harauma et al., 2007), assist in the control of blood glucose level (Kim et al., 2015a), be neuroprotective (Kang et al., 2006), prevent pneumonia (Lim et al., 2013), aid in wound healing (Kim et al., 2015b), be effective in skin whitening (Nattapong and Omboon, 2008), improve memory (Shin et al., 2010), have anticancer activity (Chen et al., 2006), be an antioxidant (Lee et al., 2014), alleviate hyperlipidemia (Jo et al., 2014), and inhibit arterial thrombosis (Lee et al., 2012).
Naowaratwattana et al. (2010) separated phenolic compounds from the organic extracts of the mulberry and identified among the constituents: rutin, isoquercetin, quercetin, and kaempferol along with the derivatives of chlorogenic acid and caffeoylquinic acid. Geng et al. (2012) also separated volatile compounds from mulberry, and identified 11 terpenes and terpenoids, 15 alkyl constituents, 26 nitrogen-containing heterocyclics, 9 oxygencontaining heterocyclics, 18 aromatics, 7 lactones, and 6 organic acids. Flavonol glucosides with antioxidant capacities were separated and identified from the water extracts of mulberry (Nam et al., 2012), and 25 physiologically active constituents including arylbenzofuran, moranin R, and albanol A were identified (Yang et al., 2011; Kikuchi et al., 2010).
Mulberry leaf have identified to contain efficacious compounds against various degenerative diseases, however, genotoxic potential and, specifically, antimutagenic effect of a MLs (Mulberry leaves) extract has not been documented. In the present study, we extracted MLs powder with 70% ethanol in order to evaluate the antimutagenic activity of MLs, and then fractionated the extracted material with diethyl ether, chloroform, dichloromethane, butanol, and water. The crude MLs ethanol extract and each of the solvent fractions was evaluated the mutagenicity using the Ames test, as well as the ability to inhibit the mutagenic effect of cigarette smoke condensates (CSCs).
MATERIALS AND METHODS
1. Mulberry leaves (MLs) source and reagents
Mulberry (Morus alba L.) leaf powder (certified by the Republic of Korea, Ministry of Food and Drug Safety) was purchased from Yakcho Wellbeing Teuku manufactured by Jaechen Hakdeul, in Ha-Myun, Gapyung-Gun, Kyunggi -Do, Korea. Reagents such as 2-aminoanthracene (2-AA) used as an indirect mutagen control, 4-nitroquinoline-noxide (4-NQ) used as a direct mutagen control, agar, Lhistidine, and D-biotin used in bacterial cell culture were from Sigma-Aldrich (St. Louis, MO, USA). All reagents and solvents used in this study were analytical grade. The S-9 mixture, a preparation containing xenobiotic metabolizing enzyme complexes of the rat liver, was purchased from the Molecular Toxicology (Boone, NC, USA) and stored at –70℃ until use.
2. Bacterial strain
The bacterial strain used in this test was Salmonella typhimurium TA98, a frameshift mutant, acquired from the Gene Bank of the Korean Research Institute of Bioscience and Biotechnology. The genotype was verified before performing each experiment. The genotype strain check was performed using the following criteria: Histidine dependence, biotin dependence, biotin and histidine dependence, presence of the rfa marker, uvr B deletion, ampicillin resistance, and spontaneous mutant frequency, according to the method described by Maron and Ames (1983).
The TA98 strain used in this test has HisD3052 allele mutation among histidine-requiring strains. This strain has enhanced sensitivity by the cell wall lipopolysaccharide (LPS), and the passage of the mutant substance is easily carried out, deep rough (rfa) mutation, uvr B deletion mutation and insertion of plasmid pKM101. Therefore, TA98 strain has more reactivity and inhibition rate than other histidine-requiring strains. After the genotype verification, the Salmonella strain was cultured overnight in Oxoid nutrient broth No. 2, before freezing at -80℃ with the addition of 0.09㎖ of the dimethylsulfoxide (DMSO) for each 1㎖ culture medium.
3. Preparation of the crude extracts and solvent extracted fractions
MLs powder, 100 g, was added to 70% ethanol solution, brought to 1ℓ, and extracted for 3 days and repeated three times. The crude extract was filtrated, concentrated by evaporation, and freeze-dried. The MLs crude extract was successively extracted with increasing polarity of solvent. Crude extracts were dissolved and sonicated in 200 ㎖ of distilled water, which was then added to the 200 ㎖ of diethyl ether for 12 - 24 h with stirring. After complete removal of the aqueous layer, the diethyl ether layer was filtered, concentrated, and freeze-dried. Chloroform was added to the aqueous layer and treated in a similar manner as the diethyl ether fractionation. In succession, a dichloromethane and butanol fractions were produced using the same procedure. Once four solvent fractions were generated from the crude MLs extract, the remaining aqueous layer was also filtered, concentrated, and freezedried. The residue from each of the four freeze-dried solvent fractions were then dissolved in dimethyl sulfoxide and the material from the aqueous layer was dissolved in phosphate buffer solution (pH 7.4) at the concentration of 40㎎/㎖. Finally, each fraction was passed through a 0.45㎛ membrane filter and stored at −80℃ until use.
4. Collection of CSCs
Forty 3R4F standard cigarettes were smoked under ISO smoking conditions using a RM-20 automatic smoking machine (Heinr Borgwaldt, Hamburk, Germany) and the following parameters: Puff volume, 35㎖; puff time, 2 min; puff duration, 1 min; butt length from the paper tip was + 3㎜. CSCs was collected via isopropanol solvent extraction by using a Cambridge filter.
5. Mutagenicity test
Mutagenicity was tested according to the method described by Maron and Ames (1983) and OECD guide line (Test no. 471) using the crude extract and each of the solvent fractions from the MLs powder.
4-NQO, a direct mutagen, a positive control, and 2-AA, an indirect mutagen, a negative control, were both used at 0.5㎍/plate in this test. The test sample treatment concentrations were 125, 250, 500, 1,000, and 2,000㎍/ plate. To each plate we added either 500㎕ of 0.2M sodium phosphate buffer (pH 7.4) in the case of direct mutagenicity test or S-9 liver mixture for the indirect mutagenicity test, which is capable of mutagenic activation of substances. For plating, Salmonella typhimurium TA 98 strain (1 - 2 × 109 cells/㎖) were suspended in 100 ㎕ culture medium and 100㎕ of test sample or control were was added in a sterilized tube, voltex-mixed for 3 - 5 s, placed on a shaking incubator for 30 min at 37℃, and then, added to 10㎖ of top agar with 1㎖ of 0.5 mM histidine plus biotin solution, voltex-mixed, spread evenly on minimal glucose agar, and cultured for 48 h in a 37℃ incubator.
The number of revertant colonies in each plate was determined using an automatic colony counter. Concurrent positive and negative controls were performed in all experiments. All testing was conducted using triplicate plates for each of the experimental concentrations.
6. Antimutagenic test
An antimutagenic test was also performed on the crude extract and each solvent fraction of MLs according to the method described by Maron and Ames (1983).
Salmonella typhimurium TA98 strain (1 - 2 × 109 cells/㎖) was suspended in 100㎕ culture medium. Separately, 200㎍ of CSCs and 500㎕ of S-9 fraction were added into a sterilized cap tube with 50㎕ of test sample, voltex-mixed for 3 - 5 s, and pre-incubated for 30 min in a 37℃ shaking incubator, after which time, the incubation mixture was added to 10 ㎖ of top agar plus 1㎖ of 0.5 mM histidine and biotin solution, voltex-mixed, spread evenly over minimal glucose agar, and cultured for 48 h in a 37℃ incubator. The number of revertant colonies in each plate was determined using an automatic colony counter. Concurrent positive and negative controls were utilized in all experiments.
All testing was conducted using triplicate plates at each of the experimental concentrations. Inhibition was calculated as follows:
where, M= number of revertant colonies by mutagen, A = number of spontaneous revertant colonies, B = number of revertant colonies when the sample and mutagen were both added to the test strains.
7. Statistical analysis
All data are presented as means ± standard deviation. Data analysis was performed using StatView version (Statview Software, Cary, NC, USA). The statistical analysis between two groups was performed using the Fisher's protected least significant difference test or Scheffe's F test. Statistical significance was defined as when the rvalue difference between two groups was less than 0.01.
RESULTS AND DISCUSSION
1. Recovery data
For the crude extract from mulberry (Morus alba L.) powder, 35.1 g of solids was recovered from 100 g of powder dissolved with 1ℓ of 70% ethanol solution (35.1%). The recovery from each solvent extraction was calculated based on the weight of crude extract fractionated by each of the four organic solvents, and after concentration.
The total yield (g/g of crude extract) for the diethyl ether, chloroform, dichloromethane, butanol, and remaining water fraction were 12.8%, 6.4%, 2.8%, 19.9% and 58.1%, respectively.
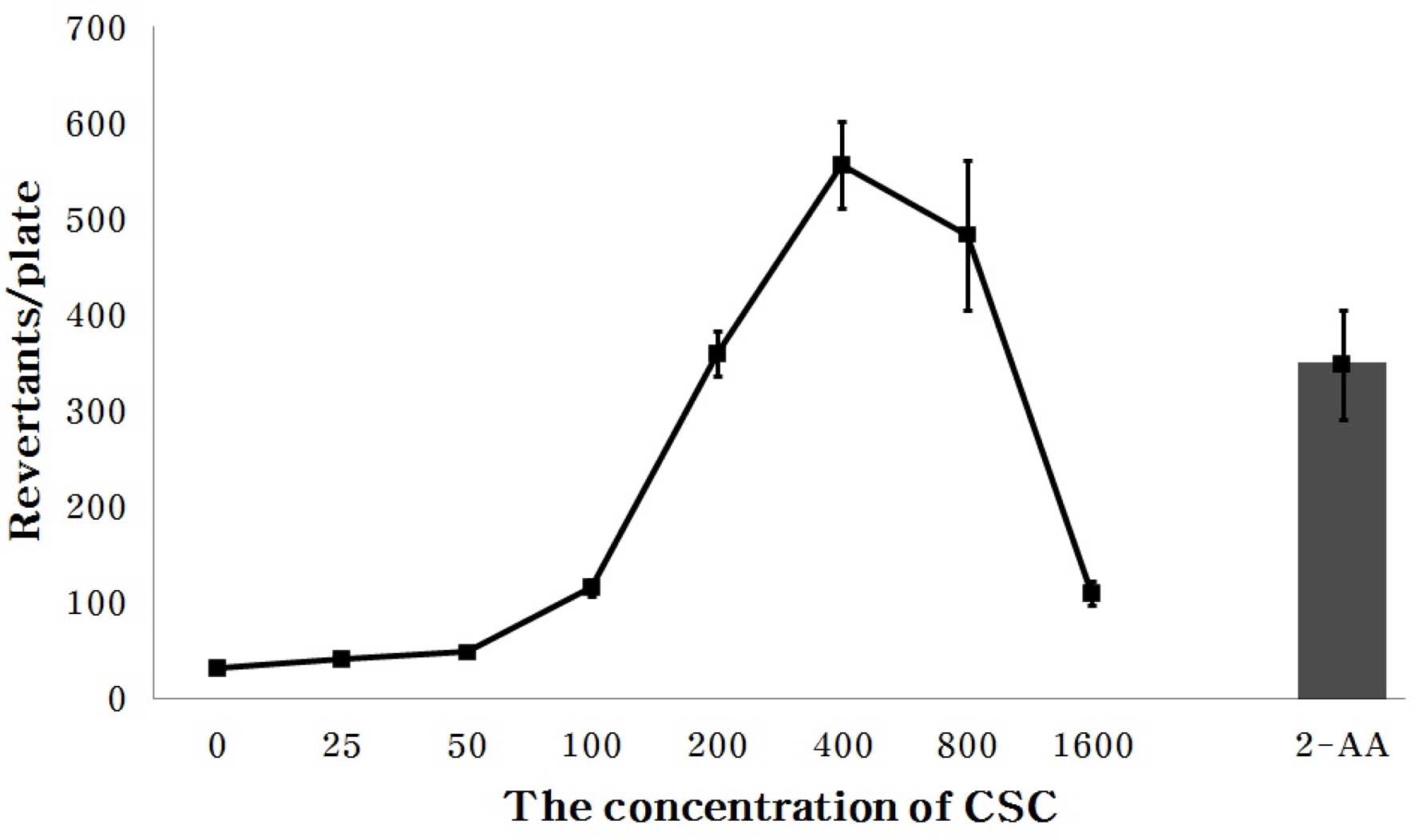
2. Selection of CSCs concentration
An appropriate CSCs (cigarette smoke condensates) concentration in culture medium for investigation of the antimutagenic effect of the crude extract and solvent extracted fractions was determined. The total amount of CSCs produced from smoking 40 3R4F standard cigarettes was 0.42 g.
Fig. 1 shows the change of revertant colony number with CSCs concentration in the culture medium inoculated with Salmonella typhimurium TA98 strain, from 25㎍/plate to 1,600㎍/plate. The revertant colony number was 31.0 ± 3.0 in the control not treated with CSCs, but was 347.3 ± 56.6 in the control treated with 2-AA (positive control). The number of revertant colonies was 41.0±3.5, 47.7±2.5, 115.3±8.2, 359.0±23.5, 555.3±44.9, 482.7±78.0 and 109.3±12.5 when CSCs were present in the culture medium at the concentrations of 25, 50, 100, 200, 400, 800 and 1,600㎍ per plate, respectively, increasing at concentrations up to 400㎍ of CSCs: Thereafter, the number of revertants decreased when CSCs were present at 800㎍ or more. One explanation is that more than 400㎍ CSCs in the culture medium may be toxic to Salmonella typhimurium TA98.
Therefore, we chose the treatment concentration of CSCs to 200㎍/plate for evaluating the inhibitory effect of the MLs crude extract and each solvent fractions against mutagenicity caused by CSCs.
3. Mutagenic activity of MLs crude extracts
In order to investigate whether MLs crude 70% ethanol extract has inherent mutagenic activity, we added the extract to the culture medium inoculated with Salmonella typhimurium TA98 at 125㎍/plate to 2㎎/plate. The results are shown in Table 1. The number of spontaneous revertant colonies in the negative control was 27.3 ± 3.5 with S-9 mix and 17.7 ± 1.5 without S-9 mix, respectively. The number of revertant colonies was 448.0±8.5 when treated the 0.5㎍ of 4-NQO, a direct mutagen, and was 524.7±10.7 when treated the 0.5㎍ of 2-AA, an indirect mutagen.
These results are consistent with those of Maron and Ames (1983), indicating our system is operating in a manner similar to published results. The number of revertant colonies did not show any significant difference compared to negative control and was not more than two times different from the negative control when MLs crude extract was tested with S-9 mix and without S-9 mix. Therefore, we conclude that MLs crude extract itself is not mutagenic to Salmonella typhimurium TA98.
4. Mutagenic activity of MLs solvent extracted fractions
We also investigated whether the MLs solvent extracted fractions have mutagenic activity. Table 2 shows the change of revertant colony number when each MLs solvent fraction was tested at 1,000㎍/plate in culture medium inoculated with Salmonella typhimurium TA98, with and without S-9 mix.
The number of revertant colonies did not show a significant difference in comparison to negative control when treated each of the diethyl ether, chloroform, dichloromethane, butanol and water extracted fractions of MLs up to 1,000㎍/plate either with or without S-9 mix. Therefore, we further concluded that none of the solvent extracted fractions of MLs crude extract has direct or indirect mutagenic activity.
5. Antimutagenic effect of MLs crude extracts
In order to investigate whether mulberry crude extract has an antimutagenic effect on CSCs, the change of revertant colony number was measured with MLs crude extract in culture medium at a concentration of 500㎍ with S-9 mix and 200㎍/plate CSCs (Table 3).
The spontaneous revertant colony number of Salmonella typhimurium TA98 was 34.3±2.5, and the number of revertant colonies was 362.4±52.5 in the presence of CSCs at 200㎍/plate in same culture medium. Moreover, the number of revertant colony was 37.3±4.8 when the MLs crude extract and CSCs were both present, giving a rate of inhibition of mutagenicity of 99.1%.
6. Antimutagenic effect of MLs solvent extracted fractions
We also investigated whether 5 each solvent fractions from MLs crude 70% ethanol extract has an antimutagenic effect on CSCs (Table 4). When treated with 1㎎/plate of material from each solvent fraction and 200㎍ of CSCs in the culture medium at the same time, the inhibition of mutagenicity against CSCs was 99.0%, 0.0%, 0.0%, 0.0% and 0.1% for the diethyl ether, chloroform, dichloromethane, butanol, and water fractions of MLs crude 70% ethanol extract, respectively.

Inhibitory effects of solvent fractions separated from MLs 70% ethanol extract against the mutagenicity of CSCs.
Therefore, the chloroform, dichloromethane, butanol, and water fractions did not show an antimutagenic effect in Salmonella typhimurium TA98 strain. Only the diethyl ether fraction of MLs extracts had an antimutagenic effect against CSCs.
7. Concentration dependence for the antimutagenic effect of the diethyl ether fraction
We further investigated the effective minimal concentration of the material in the diethyl ether fraction capable of producing an antimutagenic effect.
Table 5 shows the results of the antimutagenic assay when the diethyl ether fraction was tested at concentrations from 31.3㎍/plate to 1,000㎍/plate in culture medium inoculated with Salmonella typhimurium TA98 together with S-9 mix and CSCs at 200㎍/plate. These data confirm the antimutagenic effect of diethyl ether fraction against CSCs and showed that the effect increased as treatment concentration of diethyl ether layer increased in the culture medium. The inhibition of the mutagenicity was 97.1% in the presence of 1㎎/plate of diethyl ethyl fraction, which is approximately the same as the previous result for 500㎍/plate. The antimutagenicity data, therefore, suggest that more advanced studies such as the separation, analysis, and determination of chemical components responsible for the antimutagenic activity in the diethyl ether fraction of MLs crude extract may be needed.

Inhibitory effects on the diethyl ether fraction separated from MLs 70% ethanol extract against the mutagenicity of CSCs with concentration.
In the case of Morus alba L., Chang et al. (2016) recently reported that the fruit does not produce subchronic oral toxicity or genotoxicity at concentrations greater than 1,000㎎/㎏ in a 90 day toxicity study of using Sprague-Dawley rats. On the contrary, there are numerous reports of antimutagenic activity by various plant extracts. In one study, 800㎍ of the hexane fraction from cinnamon bark ethanol extract produced over 98% inhibition when the antimutagenic effect was evaluated against B[a]P using Salmonella typhimurium TA98 strain (Jeong et al., 1998). Other reports where Salmonella typhimurium TA98 was used, include where: 200 ㎕ of a water extract of comfrey (Symphytum officinale) showed a 50% inhibition of mutagenicity induced by B[a]P (Ham et al., 1992), 100㎍ of a butanol extracted fraction from Ixeris dentata methanol extract showed over 90% inhibition of the mutagenicity induced by B[a]P (Kim et al., 2002a), and 100㎍ of a butanol extracted fraction from Kalopanax pictus Nakai crude methanol extract showed 74.5% inhibition (Kim et al., 2002b). The inhibition of mutagenicity by 1-aminopyrene was over 90% at 15㎎ of methanol extract of Curcuma sessilis and Punica granatum flowers grown in Thailand (Wongwattanasathien et al., 2010). An extract of tea grown in Brazil inhibited 2- aminofluorene mutagenicity in the Salmonella typhimurium TA98 (Horn and Vargas, 2008). Although many of these reports used the same bacterial mutant as the present study, an accurate comparison of efficacy based on test concentrations between various plants extracts is impossible because of the differences in methods of handling and preparing the fractions of each of the medicinal herbs, and, in many cases, the percent inhibition was given without providing the data on revertant colony number.
It is known that substances that inhibit mutagenicity can be cancer suppressing due to the ability to reduce formation of DNA adducts between the mutagen and DNA (Park et al., 2016). Plant-derived compounds may also interfere with the absorption of carcinogens into the body. We propose that the constituents of the diethyl ether fraction of MLs crude 70% ethanol extract may have anticancer activity based on one of two mechanisms: first, they may be metabolically activated by xenobiotic metabolizing enzymes, such as those present in liver fractions, that react with CSCs mutagens directly: or, second, constituents in the fraction may play an important role in promoting the DNA repair system involved in removing DNA damage caused by CSCs toxins or their metabolites.
ACKNOWLEDGEMENTS
This work was carried out with the support of Chungbuk National University in 2015.
References
- Brusick, DJ, A critical review of the genetic toxicity of steviol and steviol glycosides, Food and Chemical Toxicology, (2008), 6, pS83-S91.
- Chang, BY, Kim, SB, Lee, MK, Park, H, Kim, SY, Nonclinical safety assessment of Morus alba L. fruits: Study of 90-D toxicity in Sprague Dawley rats and genotoxicity in Salmonella, Journal of Food Science, (2016), 1, p1328-1335.
-
Chen, PN, Chu, SC, Chiou, HL, Kuo, WH, Chiang, CL, Hsieh, YS, Mulberry anthocyanins, cyanidin 3- rutinoside and cyanidin 3-glucoside, exhibited an inhibitory effect on the migration and invasion of a human lung cancer cell line, Cancer Letters, (2006), 235, p248-259.
[https://doi.org/10.1016/j.canlet.2005.04.033]

-
Choi, KH, Lee, HA, Park, MH, Han, JS, Mulberry(Morus alba L) fruit extract containing anthocyanins improves glycemic control and insulin sensitivity via activation of AMP-activated protein kinase in diabetic C57BL/Ksj-db/db mice, Journal of Medicinal Food, (2016), 19, p737-745.
[https://doi.org/10.1089/jmf.2016.3665]

-
Geng, CA, Ma, YB, Zhang, XM, Yao, SY, Xue, DQ, Zhang, RP, Chen, JJ, Mulberrofuran G and isomulberrofuran G from Morus alba L.: Anti-hepatitis B virus activity and mass spectrometric fragmentation, Journal of Agricultural and Food Chemistry, (2012), 60, p8197-8202.
[https://doi.org/10.1021/jf302639b]

- Greenwell, M, Rahman, PKSM, Medicinal plants Their use in anticancer treatment, International Journal of Pharmaceutical Sciences and Research, (2015), 6, p4103-4112.
- Ham, SS, Park, GG, Park, YH, Park, WB, Antimutagenic effect of the extracts of comfrey, Journal of Korean Society of Food Science and Nutrition, (1992), 21, p539-543.
-
Han, HM, Kwon, YS, Kim, MJ, Antioxidant and antiproliferative activity of extracts from water chestnut(Trapa Japonica Flerow), Korean Journal of Medicinal Crop Science, (2016), 24, p14-20.
[https://doi.org/10.7783/kjmcs.2016.24.1.14]

-
Harauma, A, Murayama, T, Ikeyama, K, Sano, H, Arai, H, Takano, R, Kita, T, Hara, S, Kamei, K, Yokode, M, Mulberry leaf powder prevents arthertosclerosis in apolipoprotein E-deficient mice, Biochemical and Biophysical Research Communications, (2007), 358, p751-756.
[https://doi.org/10.1016/j.bbrc.2007.04.170]

-
Hong, CE, Lyu, SY, The antimutagenic effect of mistletoe lectin(Viscum album L. var. coloratum agglutinin), Phytotherapy Research, (2012), 26, p787-790.
[https://doi.org/10.1002/ptr.3639]

-
Horn, RC, Vargas, VMF, Mutagenicity and antimutagenicity of teas used in popular medicine in the Salmonella/microsome assay, Toxicology In Vitro, (2008), 22, p1043-1049.
[https://doi.org/10.1016/j.tiv.2007.12.014]

- Jeong, ET, Park, MY, Lee, JG, Chang, DS, Antimicrobial activity and antimutagenesis of cinnamon (Cinnamomum cassia Blume) bark extract, Journal of Food Hygiene and Safety, (1998), 13, p337-343.
-
Jo, SP, Kim, JK, Lim, YH, Antihyperlipidemic effects of stilbenoids isolated from Morus alba in rats fed a highcholesterol diet, Food and Chemical Toxicology, (2014), 65, p213-218.
[https://doi.org/10.1016/j.fct.2013.12.040]

- Kang, TH, Hur, JY, Kim, HB, Ryu, JH, Kim, SY, Neuroprotective effects of the cyanidin-3-O-beta-D-glucopyranoside isolated from mulberry fruit against cerebral ischemia, Neuroscience Letter, (2006), 391, p122-126.
- Khunakornvichaya, A, Lekmeechai, S, Pham, PP, Himakoun, W, Pitaksuteepong, T, Morales, NP, Hemstapat, W, Morus alba L. stem extract attenuates pain and articular cartilage damage in the anterior cruciate ligament transectioninduced rat model of osteoarthritis, Pharmacology, (2016), 98, p209-216.
-
Kikuchi, T, Nihei, M, Nagai, H, Fukushi, H, Tabata, K, Suzuki, T, Akihisa, T, Albanol A from the root bark of Morus alba L. induces apoptotic cell death in HL60 human leukemia cell line, Chemical and Pharmaceutical Bulletin, (2010), 58, p568-571.
[https://doi.org/10.1248/cpb.58.568]

- Kim, JY, Ok, HM, Kim, J, Park, SW, Kwon, SW, Kwon, O, Mulberry leaf extract improves postprandial glucose response in prediabetic subjects A randomized, double-blind placebo-controlled trial, Journal of Medicinal Food, (2015a), 18, p306-313.
- Kim, KH, Chung, WS, Kim, Y, Kim, KS, Lee, IS, Park, JY, Jeong, HS, Na, YC, Lee, CH, Jang, HJ, Transcriptomic anaysis reveals wound healing of Morus alba root extract by up-regulating karatin filament and CXCL 12/CXCR4 signaling, Phytotherapy Research, (2015b), 29, p1251-1258.
- Kim, MJ, Kim, JS, Jeong, DM, Ham, SS, Yu, CY, Effect of antioxidant, antimutagenicity and anticancer of root extract from Ixeris denate Nakai, Korean Journal of Medicinal Crop Science, (2002a), 10, p222-229.
- Kim, MJ, Kim, JS, Kang, WH, Yeon, KD, Antimutagenic and cytotoxic effects of extracts of Kalopanax pictus Nakai endodermis, Korean Journal of Medicinal Crop Science, (2002b), 10, p132-138.
- Lee, JJ, Yang, H, Yoo, YM, Hong, SS, Lee, D, Lee, HJ, Lee, HJ, Myung, CS, Choi, KC, Jeung, EB, Morusinol extracted from Morus alba inhibits arterial thrombosis and modulates platelet activation for the treatment of cardiovascular disease, Journal of Atherosclerosis and Thrombosis, (2012), 19, p516-522.
-
Lee, JS, Kim, YR, Park, JM, Ha, SJ, Kim, YE, Baek, NI, Hong, EK, Mulberry fruit extract protects pancreatic β-cells against hydrogen peroxide-induced apoptosis via antioxidative activity, Molecules, (2014), 19, p8904-8915.
[https://doi.org/10.3390/molecules19078904]

-
Lee, YJ, Choi, DH, Kim, EJ, Kim, HY, Kwon, TO, Kang, DG, Lee, HS, Hypotensive, hypolipidemic, and vascular protective effects of Morus alba L. in rats fed an athergenic diet, The American Journal of Chinese Medicine, (2011), 39, p39-52.
[https://doi.org/10.1142/s0192415x11008634]

-
Lim, HJ, Jin, HG, Woo, ER, Lee, SK, Kim, HP, The root barks of Morus alba and the flavonoid constituents inhibit airway inflammation, Journal of Ethnopharmacology, (2013), 149, p169-175.
[https://doi.org/10.1016/j.jep.2013.06.017]

-
Maron, DM, Ames, BN, Revised methods for the Salmonella mutagenicity test, Mutation Research / Environment Mutagenesis and Related Subjects, (1983), 113, p173-215.
[https://doi.org/10.1016/0165-1161(83)90010-9]

-
Nam, S, Jang, HW, Shibamoto, T, Antioxidant activities of extracts from teas prepared from medicinal plants, Morus alba L., Camellia sinensis L., and Cudrania tricuspidata, and their volatile components, Journal of Agricultural Food Chemistry, (2012), 60, p9097-9105.
[https://doi.org/10.1021/jf301800x]

-
Naowaratwattana, W, De-Eknamkul, W, De Mejia, FG, Phenolic-containing organic extracts of mulberry(Morus alba L) leaves inhibit HepG2 hepatoma cells through G2/M phase arrest, induction of apoptosis, and inhibition of topoisomerase Iiα activity, Journal of Medicinal Food, (2010), 13, p1045-1056.
[https://doi.org/10.1089/jmf.2010.1021]

-
Nattapong, S, Omboon, L, A new source of whitening agent from a Thai mulberry plant and its betulinic acid quantitation, Natural Product Research, (2008), 22, p727-734.
[https://doi.org/10.1080/14786410601130794]

-
Park, JH, Jang, TW, Lee, SH, Antioxidative activities and inhibition effects on oxidative DNA damage of Valeriana fauriei, Korean Journal of Medicinal Crop Science, (2016), 24, p464-470.
[https://doi.org/10.7783/kjmcs.2016.24.6.464]

- Shin, PH, Chan, YC, Liao, JW, Wang, MF, Yen, GC, Antioxidant and cognitive promotion effects of anthocyanin-rich mulberry(Morus atropurpurea L) on senescence-accelerated mice and prevention of Alzheimer's disease, The Journal of Nutritional Biochemistry, (2010), 21, p598-605.
-
Wongwattanasathien, O, Kangsadalampai, K, Tongyonk, L, Antimutagenicity of some flowers grown in Thailand, Food and Chemical Toxicology, (2010), 48, p1045-1051.
[https://doi.org/10.1016/j.fct.2010.01.018]

-
Yang, ZG, Matsuzaki, K, Takamatsu, S, Kitanaka, S, Inhibitory effects of constituents from Morus alba var. multicaulis on differentiation of 3T3-L1 cells and nitric oxide
production in RAW264.7 cells, Molecules, (2011), 16, p6010-6022.
[https://doi.org/10.3390/molecules16076010]