Effects of Polyethylene Glycol Priming on Seed Germination of the Medicinal Plant, Kenaf
© The Korean Society of Medicinal Crop Science. All rights reserved.
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License ( http://creativecommons.org/licenses/by-nc/3.0 ) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The present study assessed the response of kenaf (Hibiscus cannabinus L., Jangdae) seed to NaCl and the effects of polyethylene glycol (PEG) on kenaf seed germination and vigor..
Seed germination ranged from 11.3% to 58.8% after 24 hours of immersion in NaCl concentrations from 0% to 0.5%. The priming treatments had lower electrical conductivity (EC) values for the seeds than for the control and a deteriorated palisade layer. Priming in 10% PEG for 48 hours increased the germination upto 96.3% in H2O solution and 98.8% in 0.3% NaCl solution compared to that of the control (78.8%). Germination synchronization, and shoot and root growth of the primed seeds were greater than those of the control. The T50 of the control in H2O and 0.3% NaCl solution was 22 and 28 times, respectively. After priming, nine times was sufficient to reach T50 in both solution. The mean number of days to germination (MDG) decreased from 1.43 days for the control to 0.55 days for 0% PEG in H2O solution and from 1.57 days for the control to 0.56 days for 0% PEG in 0.3% NaCl solution. The dry weight after the 10% PEG treatment was higher than that of the control.
Taken together, 10% PEG treatment for 24 hours is recommended for kenaf seed invigoration before planting.
Keywords:
Hibiscus cannabinus L., Priming, Polyethylene Glycol, Germination PercentageINTRODUCTION
Kenaf (Hibiscus cannabinus L.) from the family Malvaceae is a common warm season annual fiber plant native to India and Africa (Yazan et al., 2011). Although kenaf is a tropical plant, today its cultivars are well adapted to a wide geographical and climatic range (Danalatos and Archontoulis, 2010).
Kenaf plants have been widely used for the production of paper, biocomposites, fiber boards and bioplastics and in the textile industry. It is an important cordage crop in many developing countries. In developed countries such as USA and Japan, it has been grown for fiber production and forage. Also, various biologically active compounds have been reported in the kenaf seed, including omega-3 fatty acids, phenolic compounds, and sterols (Alexopoulou et al., 2013; Ryu et al., 2013). Phenylpropanoids abundant in the kenaf leaf are important for these beneficial health effects (Jin et al., 2013; Ryu et al., 2016).
Kenaf was introduced in Korea in the 60’s bur ever since, it has limited use in the country. In resent years, its value in Korea has been increasing as forage and biomass production for fuel. Kenaf can reach heights of 4 - 6 m depending on environmental conditions. It requires 50 - 140 days to complete maturity, so seed production is limited to frost-free areas for the late ripening variety. Its stalk is composed of two distinct fibers, bast and core, which comprise approximately 35% and 65% of the stalk mass, respectively (Columbus and Fuller, 1999).
Bast is characterized as a bark, containing long fibers, and the core being physically similar to balsawood, containing soft, short fibers. They sharply lose germination capacity after harvest because of the high oil content of its seeds. Under conditioned storage at 20℃ and 10% humidity, kenaf remained viable for about 8 months (Carberry et al., 1990).
However, under the hot and humid climates with average ambient temperature around 35℃ and humidity above 60%, its viability loss is faster (Daniel et al., 2012). There is a kenaf variety called ‘Jangdae’ released from KAERI (Korea Atomic Energy Research Institute). It has a low germination rate which is a major limitation to commercially use in Korea.
The reclaimed tidal land of Korea is 135,100 ㏊ which is about 9% of cultivation acreage (Lee et al., 2012). Various approaches have been carried out to increase the availability of land and collection of plants suitable for the reclaimed tidal land (Lee et al., 2000; Oh et al., 2015). From their results, there were some problems in plants containing a high C/N ratio posing nitrogen starvation which consequently leads to relatively low yield. However, kenaf has a relatively low C/N ratio of 30 - 60 and a higher yield of roughly 17 - 32 t/㏊ on the dry basis (Wang, 1994). Those are the advantages of kenaf over other plants.
Rapid and uniform field emergences of seeds are two essential pre-requisites to increase yield, quality and ultimately profit in annual crops. Slow germination ability of some seeds results to smaller seedlings and consequently smaller plants. This also makes such seedlings more vulnerable to soil-born diseases (Bennett, 1998).
Uniform performance of seeds of cultivated plants is seldom achieved, where a seed represents an amalgam of individuals, each with different germination vigor (McDonald, 2000). For these reasons, seed priming has become a common treatment to increase the rate and uniformity of emergence in many vegetable and flower species, where it results in a more rapid and uniform germination when the seeds are re-imbibed (Gurusinghe et al., 1999).
Seed priming has been used to accelerate germination, uniform seedling emergence and improve germination performance under the temperature or drought stresses (Janmohammadi et al., 2008; Jahangir et al., 2009).
Seed osmopriming using either PEG had improved the germination potential of some herbaceous perennials (Finnerty et al., 1992), pigeonpea (Nayyar and Malik, 1993), brassica (Rao and Phillips, 1993), and carrot (Duman and Esiyok, 1998). However, the secret to successful seed priming is ceasing the priming treatment at just the right time to allow re-drying, hence each species must be investigated for optimal priming treatments and treatment durations (Bradford, 1986).
Therefore, the objectives of this trial were to investigate the effects of PEG on kenaf seed (Jangdae) germination and vigour, and optimize the condition with PEG concentration.
MATERIALS AND METHODS
1. Seed and other materials preparation
The kenaf (Hibiscus cannabinus L.) variety was Jangdae released from KAERI (Korea Atomic Energy Research Institute). The experiment was conducted in the Laboratory of Agri-Food Processing, Agricultural Research and Extension Services, Iksan from February to April, 2017.
Seeds, 12 g for each treatment were sterilized by soaking in NaOCl solution for 10 min and were dried for 30 minutes. Containers, with brand name Twist pack Ⓐ 400 (Twist pack 3, ACE Industry Inc., Norcross, GA, USA), were purchased at a store.
2. Measurement of seed water uptake and electrical conductivity value
To determine the water uptake, three replicates of 12 g seed samples were soaked at water (150㎖) and kept seeds in an incubator (HB-302S-4, Hanbaek Scientific Co., Bucheon, Korea) at 25℃. The moisture content was measured after 1, 3, 6, 12 and 24 hours.
To determine the electrical conductivity (EC) value, seeds were treated with various PEG concentration (0, 10, 20, 25, 30, 35, and 40%) for 24 hours at constant temperatures of 20℃ in the dark in an incubator (HB- 302S-4, Hanbaek Scientific Co., Bucheon, Korea), and its value was measured in 1, 3, 6, 12 and 24 hours using an EC measuring instrument (D-74G, HORIBA Jobin Yvon, Kyoto, Japan).
3. Germination tests to NaCl and PEG
To confirm RD50, salt concentration causing 50% growth reduction, of kenaf seeds to salt, prepared seeds were inoculated in various NaCl solutions (0, 0.1, 0.2, 0.3, 0.4, and 0.5%) for 72 hours.
The solutions were used for seed germination and distilled water was used as the control. Three replicates with 20 seeds each were placed in covered Twist pack Ⓐ 400 (Twist pack 3, ACE Industry Inc., Norcross, GA, USA) container with two filter paper soaked with 7㎖ test solutions. Seeds were germinated at constant temperatures of 25℃ with lights on an incubator (HB- 302S-4, Hanbaek scientific Co., Bucheon, Korea).
Germination was scored at same time daily. A seed was considered to be germinated as seed coat ruptured, plumule and radicle came out and were > 2㎜ long. Plant, three replicates of 10 plants, was dried at constant temperatures of 60℃ for 2 days and dry weight was scored.
In the conduct of PEG (poly ethylene glycol 6000, Sigma-Aldrich Co., St. Louis, MO, USA) test, which was used as osmopriming agent solution, seeds were primed with various PEG concentration (0, 10, 20, 25, 30, 35, and 40%) for 24 hours at constant temperatures of 20℃ in the dark in an incubator (HB-302S-4, Hanbaek Scientific Technology Co., Ltd., Bucheon, Korea), three replicates with 20 seeds each were placed in Twist pack Ⓐ 400 (Twist pack 3, ACE Industry Inc., Norcross, GA, USA) container with two filter paper soaked with 7㎖ test solutions, and germinated for 72 hours at constant temperatures of 25℃ with lights on a previous incubator.
Germination was scored at same time daily. Plant, three replicates with 10 plants each, was dried at constant temperatures of 60℃ for 2 days and dry weight was scored.
4. Measurement of growth degree of shoot and root to salt
Shoot and root growth were evaluated for 5 days after germination. All seedlings were taken from each treatment and growth was observed using the naked eye. Root and shoot length were not measured due to severe distortion.
5. Germination performance measurements
At the end of experiment, germination rates, growth of shoots and roots in NaCl solutions, T50 (times to reach 50% of the final germination rate), MDG (mean number of days to germination), and dry weight were recorded to evaluate germination performance. Dry weight was measured using a four digit balance and expressed in gram.
6. Scanning electron microscope image
The seeds were air-dried for 48 hours at 30℃. Each was then fastened using a nipper before cutting vertically to avoid mechanical injury for the vertical section filming. The specimen were air-dried for 20 hours at 80℃ to shoot, then was placed on aluminum stub and plated with gold by a gold ion sputtering device (JFC-1100E, Fine Coat, Jeol Ltd., Tokyo, Japan) at 10㎃ for 400 seconds.
A palisade layer of seeds plated with gold were observed using a scanning electron microscope (JSM- 5410LV, Jeol Ltd., Tokyo, Japan) at condition of 15㎸.
7. Statistics
Results were analyzed for analysis of variance (ANOVA) using SAS Enterprise Guide 4.2 (Statistical Analysis System, 2009, SAS Institute Inc., Cary, NC, USA). Means were compared at 5% significance level using Duncan’s Multiple Range Test (DMRT) comparison (p < 0.05 and 0.01).
RESULTS AND DISCUSSION
1. Response of kenaf to NaCl
The first aim of this study was to assess the response of kenaf (Hibiscus cannabinus L.) seed at various NaCl levels. In this part of the study, kenaf seeds was only exposed to 0% NaCl (NaCl-free, NF), and eventually to other NaCl concentrations (Curtis and Lauchli, 1986).
The germination percentage of seed ranged from 11.3 to 58.8 after 24 hours and from 66.3 to 86.3 after 72 hours, respectively (Fig. 1). There was a significant difference in germination percentage between seeds grown on solution with NF and 0.1% NaCl after 24 hours exposure. However, there was no difference between 0.4% NaCl treatment compared to NF after 72 hours. Only, a meaningful distinction in 72 hours was shown at 0.5% NaCl treatment (Fig. 1).
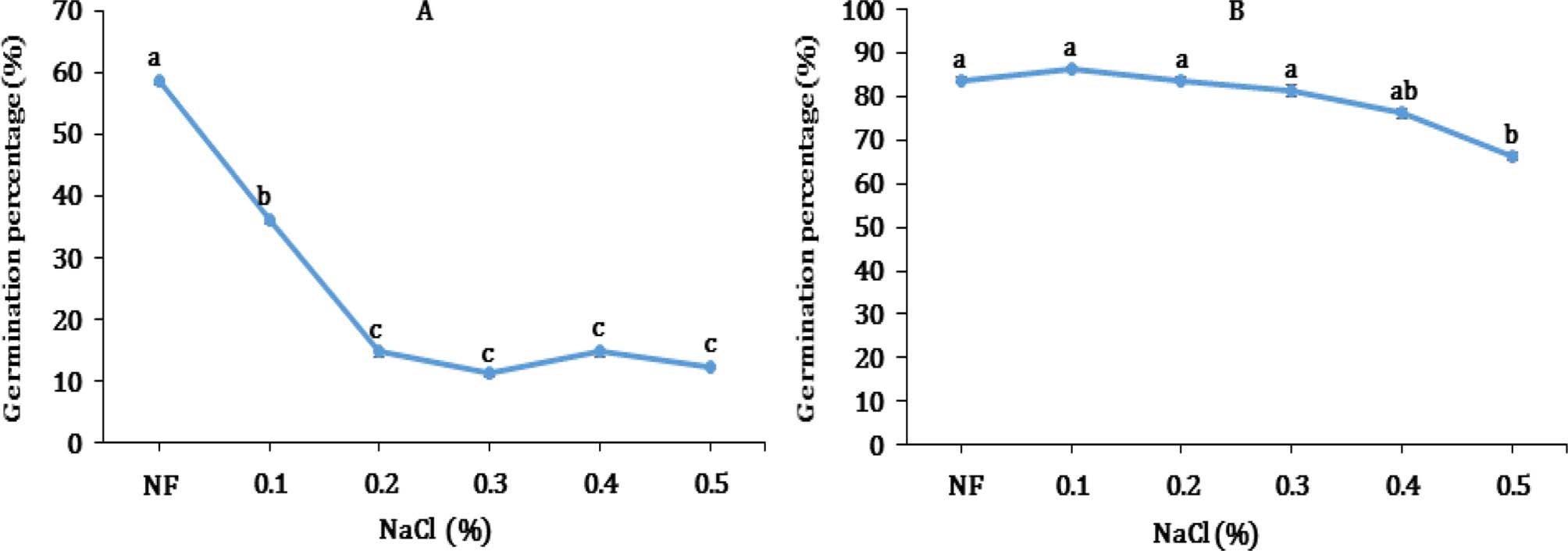
Response in germination of kenaf seed at different NaCl concentration after 24 (A) and 72 (B) hours of immersion, respectively.Data represent the means ± S.E of three replicates. *Different letter for each treatment shows significant difference (p < 0.01) using Duncan’s Multiple Range Test (DMRT) comparison. NF; NaCl-free, indicates seeds grown on solution without NaCl.
Bases on this observation, it is concluded that kenaf was placed in the category of moderate salt tolerant nonhalophytic crop under salinity. This result was in agreement with that of an earlier report (Mass and Hoffman, 1977). Previous study showed that its germination percentage declined with increasing salt concentration (Francois et al., 1990). Curtis and Läuchli (1985) noticed that seed germination of kenaf cultivars were only slightly impaired by NaCl salinity upto 200 mM.
The results of kenaf shoots and roots growth difference are presented in Table 1. Its vegetative development decreased as the salt stress level increased. The RD50, salt concentration of causing 50% growth reduction, for shoots and roots growth is observed at 0.2 - 0.3% NaCl. Curtis and Curtis and Läuchli (1986) reported a threshold of about 0.21% NaCl for significant growth reduction of kenaf due to salinity. We took up 0.3% of NaCl as selection concentration for subsequent experiment. Leaf growth rate declined linearly with increasing salt stress (Curtis and Läuchli, 1985).

Growth difference of kenaf shoots and roots at various NaCl concentrations after 5 days of germination.
Plant dry weight was approximately 0.2 g in all salt treatments after 5 days of seed inoculation (Fig. 2). Based on this result, a kenaf dry weight was not negatively impacted by NaCl concentration upto 0.5%. It is concluded that a dry weight is not suitable criteria to estimate salt tolerance of kenaf upto 5 days. So, a longer duration of the test is needed to be able to use this criteria to estimate salt tolerance of kenaf.
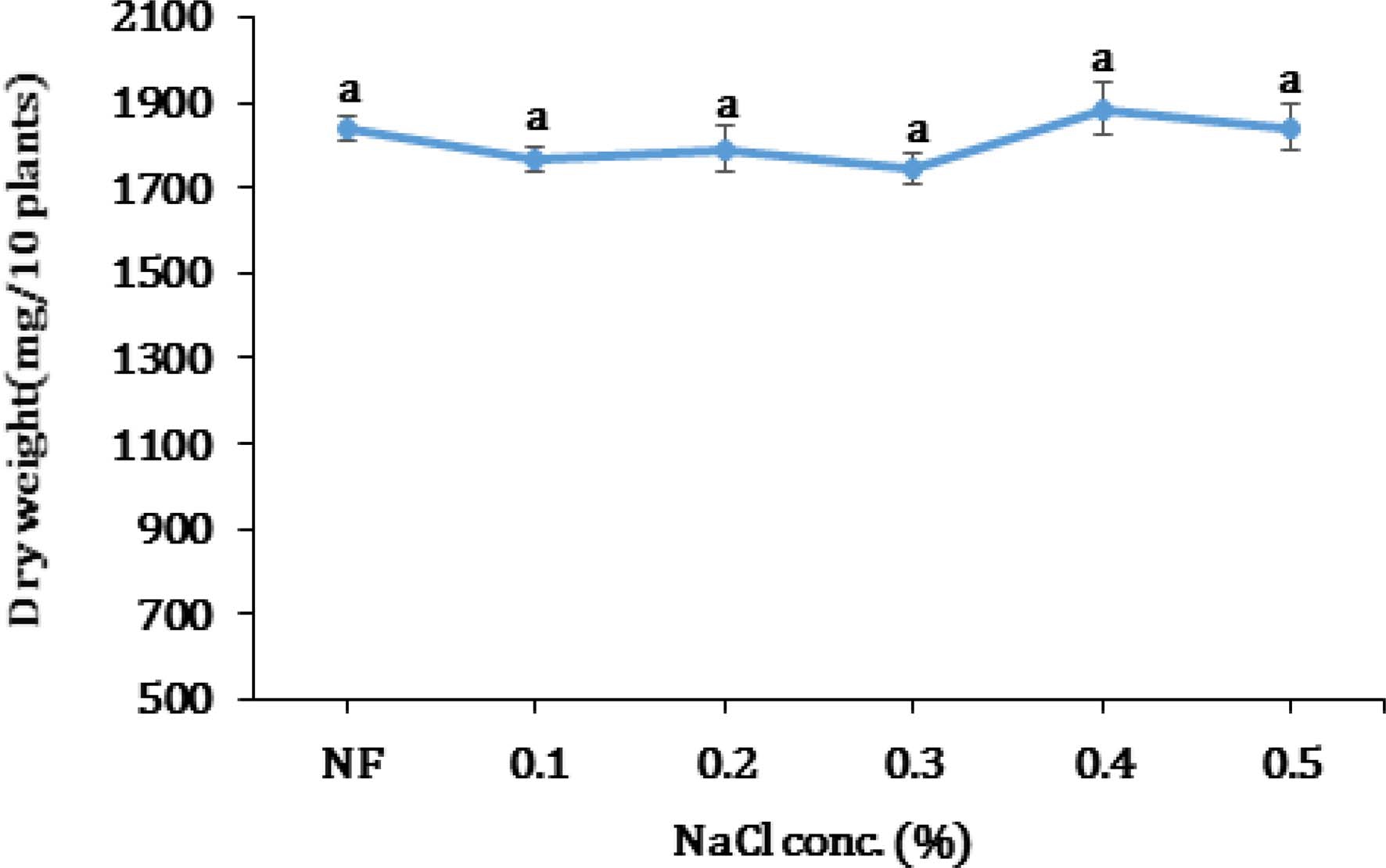
Kenaf plant dry weight at various NaCl concentrations after 5 days of germination.concentrations after 5 days of germination. Data represent the means ± SE of three replicates per replicate for each of the 10 plants. *The same letter for each treatment shows no significant difference using Duncan’s Multiple Range Test (DMRT) comparison. NF; NaCl-free, indicates seeds grown on solution without NaCl.
Francois et al. (1992) reported that the dry weight of stem was only reduced 11.6% in soil salinity above 8.1 ds/ m, which is comparable to 0.42% NaCl. After comparing the results presented in Fig. 1 and 2, we concluded that germination was a more sensitive tool in measuring the effect of salinity to kenaf rather than dry weight.
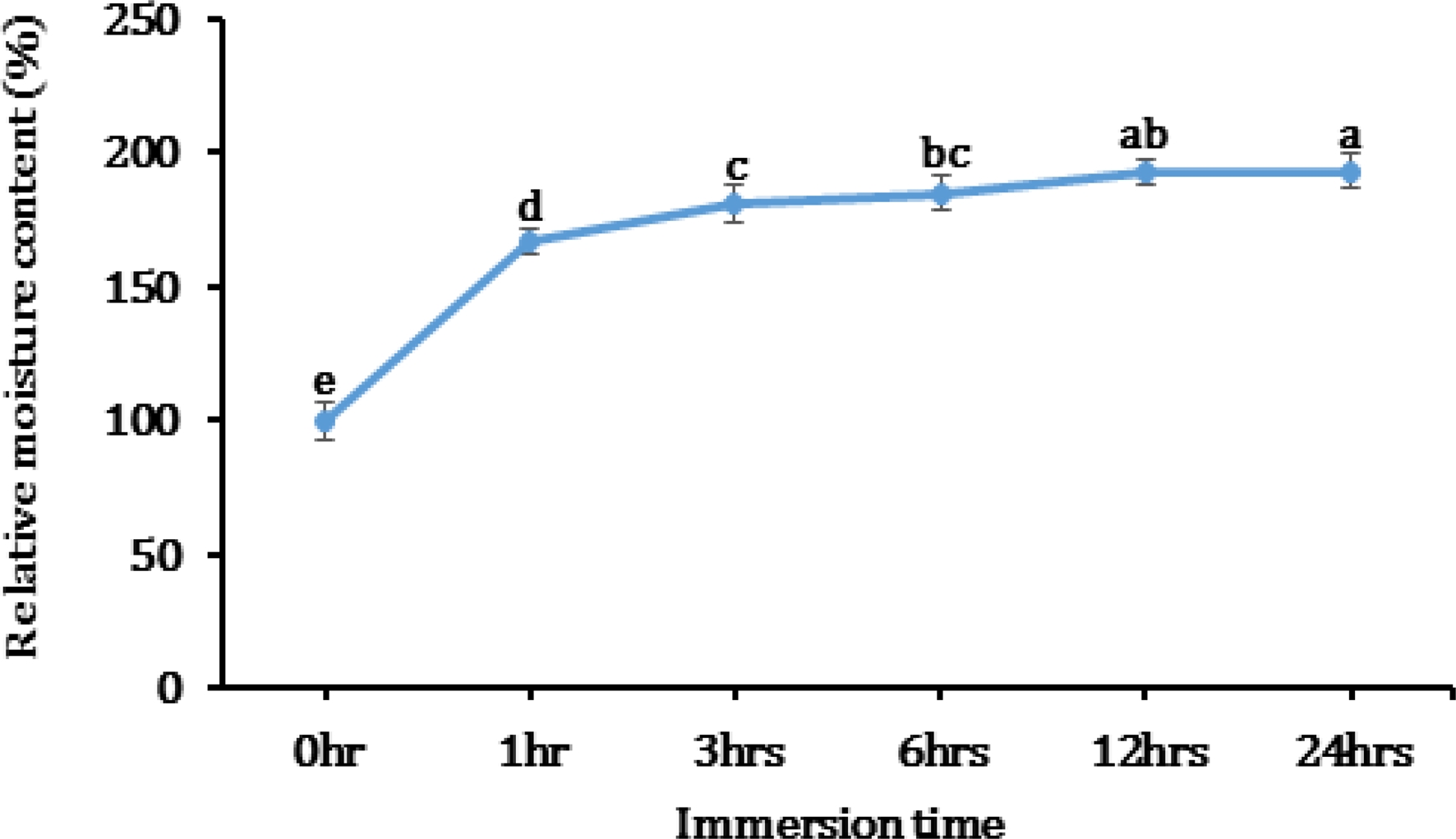
2. Effect of various immersion times on seed water uptake
Kenaf is an upland-field crop. Thus, prolonged immersion of its seeds in water caused a decrease in vitality and shortens the seed’s life, resulting to germination percentage reduction. It is important to determine the optimum immersion period for preventing water absorption by a balance of water potential.
Before priming treatment, seed moisture was about 10%. As the immersion times increases, seed moisture gently increased up to 1.93 times after 24 hours. Priming treated seeds, particularly for 3 hours, had rapid water uptake at the early times of immersion. Beyond 12 hours, its content was statistically same. Thus, it implies that the critical point for soaking kenaf seed in water is 12 hours. After 12 hours, a part of the seeds broke seedcoat by coming out radicle (Fig. 3). Similar result was observed by Shekari et al. (2015).
3. Effect of various PEG and immersion times on ion leachate
In this part of the study, kenaf seeds were only exposed to 0% PEG and was expressed as HP (hydropriming) (Table 2, Fig. 4).
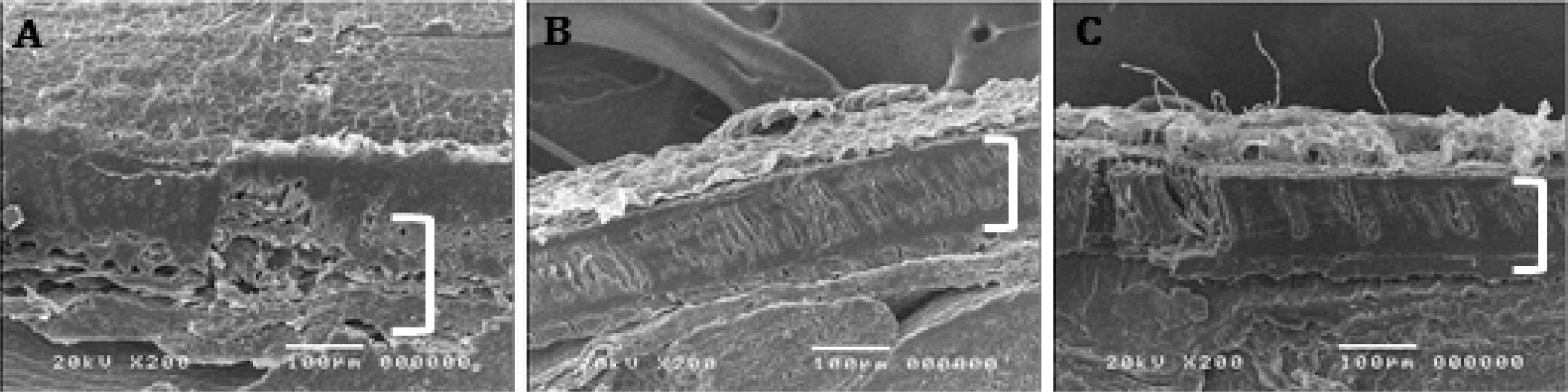
Scanning electron microscope image of cross section of kenaf seed coat after PEG priming treatment for 24 hours.A; control indicates seeds untreated with priming, B; HP indicates seeds grown on solution without PEG was expressed as HP (hydro-priming), C; 10% PEG treatment. A white parenthesis indicates palisade layer.
As times went by, electrical conductivity (EC) value of HP increased consistently. In the presence of PEG (10 - 40%), the ion leakage concentration of the solution decreased sharply compared to HP (Table 2). HP treatment brought about higher palisade layer breakage than the PEG treatment. As a result, plenty of minerals were released through the impaired tissues, thus increasing the EC value. The EC increase signifies the degree of loss of solutes from the seeds, which reflects the extent of membrane deterioration resulting from seed aging (Roberts, 1989). By adding PEG, EC value decreased. Its value was observed to decrease starting from 10% PEG. It is clear that an alteration of EC value was affected by water rather than PEG. The priming treatments (hydro-priming and PEG) deteriorated palisade layer (Fig. 4B, C).
Kang and Choi (2006) who reported that the PEG priming treatment increased the leakage of inorganic compound such as Ca2+ and K++ from hot pepper seed, which is in accordance with our result. Also, Kang et al. (2017) stated that the physicochemical properties of Sancho oil were changed according to purification process, which is caused by a deterioration of cell wall.
4. Effect of PEG on surging the germination percentage
Germination of Jangdae seed was generally poor in the control condition based on the kenaf seed maturity. So, it is necessary for kenaf seed to apply priming technique like PEG so as to surge germination uniformity and capability.
Table 3 illustrates the final germination against time, respectively. At the first 12 hours of treatment, the maximum germination rate was 78.8% in H2O solution and 86.3% in 0.3% NaCl solution after 10% PEG treatment. However, control’s (seed unprimed with HP and PEG) germination percentage showed 0% under both solutions. The highest germination was recorded between 24 and 48 hours under H20 and 0.3% NaCl solutions.
At 72 hours, the earlier germinated seedlings died from fungal infection, resulting to a decrease of germination under both solutions. It is then concluded that 10% PEG was the critical concentration to surge germination. This method considerably reduced the time required to initiate seed germination.
Kim et al. (2006) reported that the pasture seed germination increased at 10 - 30% PEG treatment. These results are attributed to chemical effects of PEG causing cell wall looseness thus increasing water uptake of cells.
The most probable mechanism for PEG enhancement of germination is the intensification of mass transfer and easier access of the water to the interior of the cell wall structure.
As to findings at 72 hours, it was represented with a survival seedlings. Significant difference in survival rate of seedlings also occurred among treatments for both solutions. In H2O solution, the survival rate of 90% at 20 and 25% PEG treatment were significantly greater than that of the other treatments.
Also, the survival rate of 95% at 10% PEG treatment was significantly greater than that of the other treatments under 0.3% NaCl solution. The results can be contributed to a lower EC value caused by minimal tissue damage (Table 2). A high EC value in the solution indicates high minerals leakage from the seeds thus affected the survival rate at 72 hours.
Ismail et al. (2006) stated that a higher germination percentage was obtained with PEG treatment compared to the control. PEG would be the preferred osmoticum because it is inert and the embryo can not take up its large molecular size (McDonald, 2000). PEG’s molecular hinder too much water absorption by seed. Excessive water absorption by the seeds hampers seed germination.
Also, Davison and Bray (1991) suggested that PEG treatment induced various proteins synthesis related with germination. The earlier germinated seedlings after priming treatment such as HP and 10% PEG showed earlier dead. Previous studies on cotton and kenaf have shown a strong association between rapid germination and seedcoat susceptibility to mold growth, seed rot and diseased seedling roots (Bird, 1982; Cook et al., 1992).
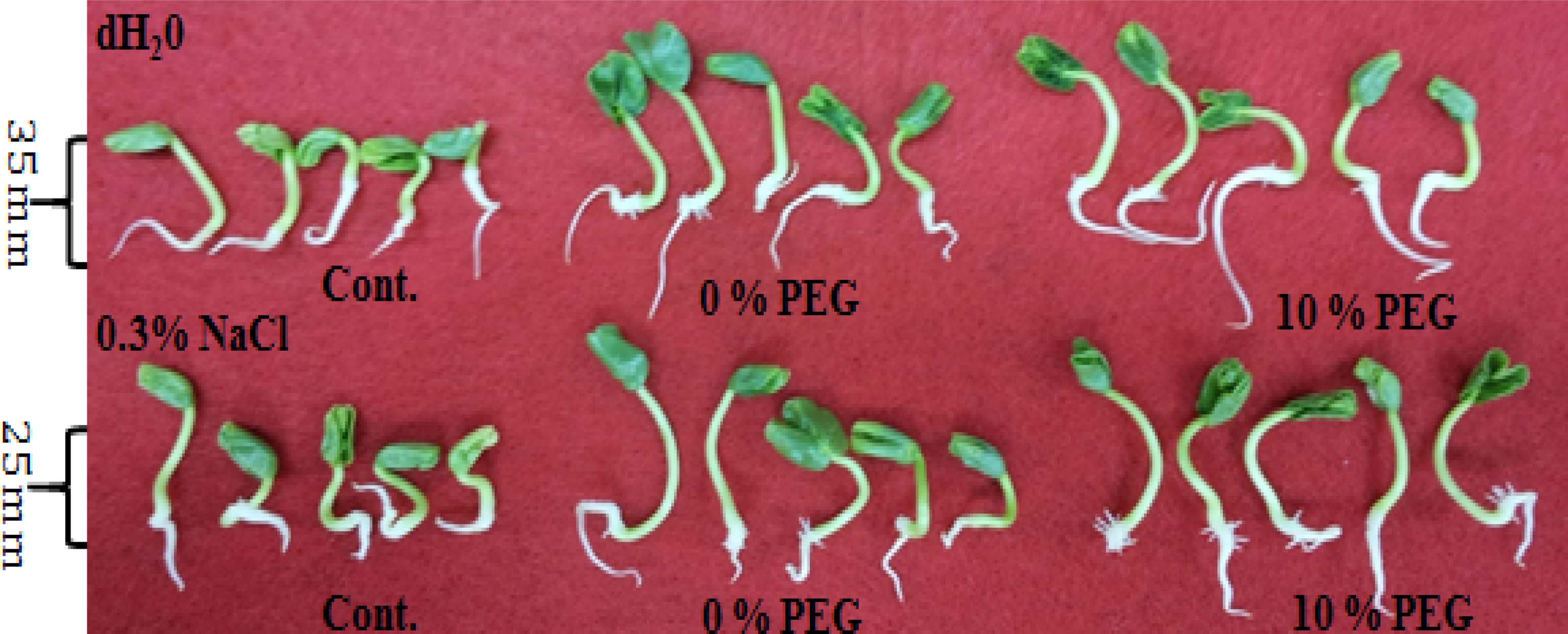
Germination synchronization, shoot length, and leaf unfolding of primed seed were greater than those of the control. Also, main root or hair root appeared faster in the treated seeds and grew abundantly compared to the control (Fig. 5). Enhanced effect, which hasten germination rate and growth progress was greatest at HP and 10% PEG under both solutions compared to the control. (Fig. 5).
5. Effect of PEG on surging both T50 and MDG
The findings of T50 (times to reach 50% of the final germination rate) and MDG (mean number of days to germination) after PEG treatment is as follows.
The T50 of the control under H2O and 0.3% NaCl solution was 22 and 28 times, respectively. At HP and 10% PEG treatment, 9 times was sufficient to reach T50. It is over 2 - 3 times higher than that of control in H2O and 0.3% NaCl solutions (Table 4). T50 of the PEG treatments were higher than that of the control. MDG decreased from 1.43 days being maximum for the control. to 0.55 days being minimum for HP in H2O solution and from 1.57 days being maximum for the control to 0.56 days being minimum for HP in 0.3% NaCl solution (Table 4).
The analysis of the resulting data indicates that the T50 and MDG were significantly affected by the PEG priming during the germination. A previous study demonstrated that an increase in germination rate and MDG in primed barley seeds may be due to initiating metabolic events in primed seeds (Yaldagard et al., 2008).
Li et al. (1999) reported that the seed primed with PEG showed a fast MDG, resulting to improved germination rate rather than an increase in germination percentage. It appeared that PEG treatment might induce various proteins synthesis related with germination (Davison and Bray, 1991). Fig. 5
6. Effect of PEG on the dry weight
Results indicated an effect of priming on dry weight at 3 days after initiating germination (Fig. 6). In H2O solution, the dry weight was similar in all test treatments (1,867 - 1,943㎎/10 plants) except at 10% PEG with 2,088 ㎎/10 plants which was noticeably higher. There was no significant difference on dry weight observed between the control and primed seeds exposed to abiotic stress of 0.3% NaCl.
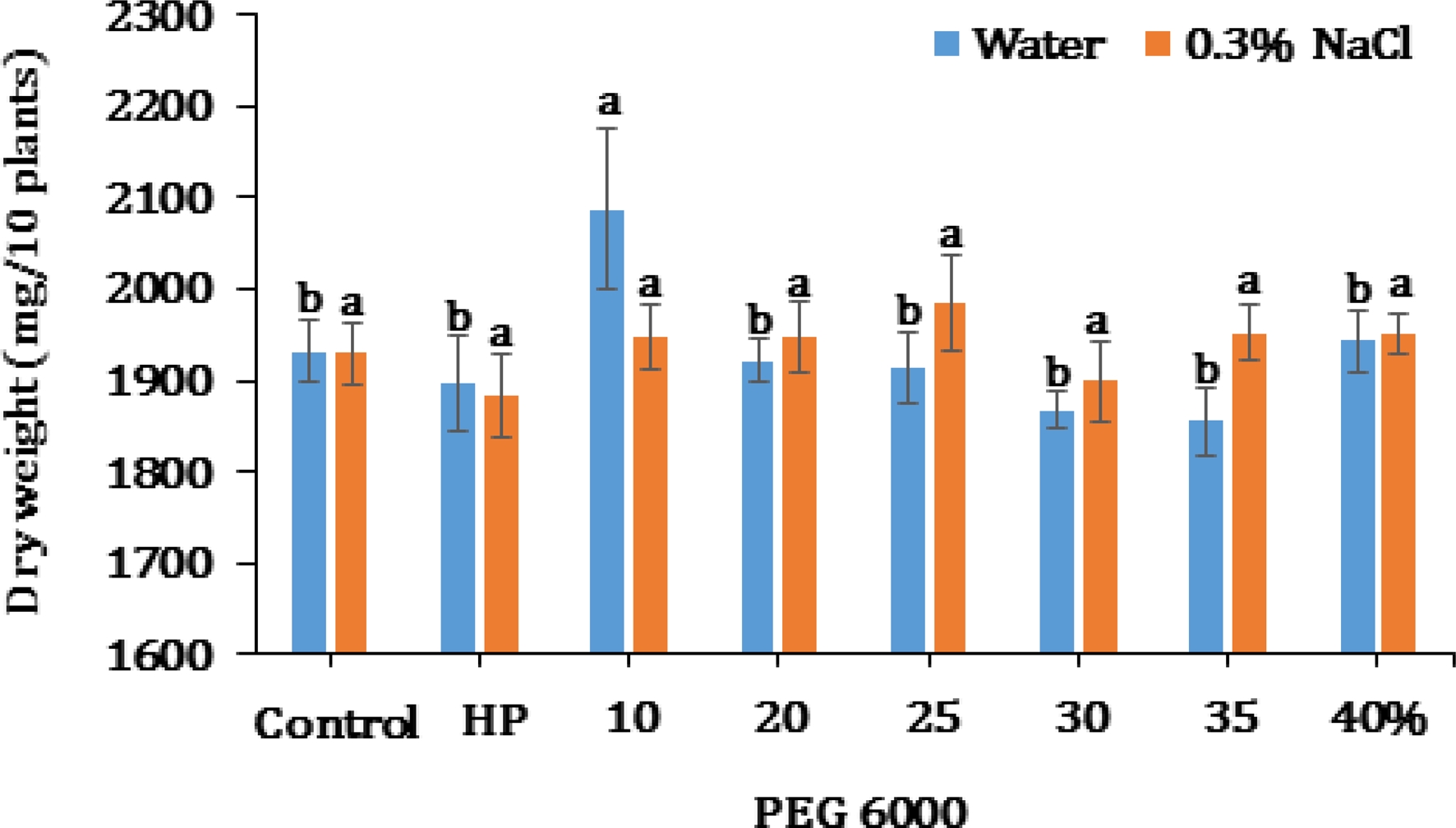
Dry weight of seedling at various PEG concentrations after 3 days of germination under H2O and 0.3% NaCl.Data represent the means ± S.E of three replicates per replicate for each of the 10 plants. *Different letter for each solution shows significant difference (p< 0.05) using Duncan’s Multiple Range Test (DMRT) comparison. Control indicates seed unprimed with HP and PEG. HP indicates seed primed with hydro-priming.
However, the dry weight measurement after the PEG priming treatment showed to be higher compared to the control. According to Ismail et al. (2005), the dry weight of tomato after PEG treatment soared. Our results concur with their findings. It seemed that there is a close connection between PEG treatment and dry weight.
In conclusion, the study performed on kenaf suggested that its seeds can be primed with both hydropriming (water) and osmopriming (PEG) to boost germination. Osmopriming with 10% PEG for 24 hours could significantly improve results rather than non-priming and hydropriming regardless of abiotic stress based on the germination rate, T50, and MDG. Finally, we are recommending this technique to improve the economic yield of kenaf.
We primed kenaf seeds for 24 hours following the methodology in Daniel et al. (2012). However, further research is needed to reduce durations of the priming treatment to decrease the operation costs.
ACKNOWLEDGEMENTS
This work was carried out with the support of Cooperative Research Program for Agriculture Science and Technology Development(PJ011968) Rural Development Administration, Republic of Korea.
References
- E. Alexopoulou, Y. Papattheohari, M. Christou, A. Monti, Keanf: A multi-purpose crop for several industrial applications, London, England, Springer-Verlag London, (2013), p1-15.
- M.A. Bennett, The use of biologicals to enhance vegetable seed quality., Seed Technol, (1998), 20, p198-208.
- L.S. Bird, The MAR(multi-adversity resistance) system for genetic improvement of cotton., Plant Dis, (1982), 66, p172-176.
- K.J. Bradford, Manipulation of seed water relations via osmotic priming to improve germination under stress conditions., HortScience, (1986), 21, p1105-1112.
-
P.S. Carberry, D.G. Abrecht, Germination and elongation of the hypocotyls and radicle of kenaf(Hibiscus cannabinus) in response to temperature., Field Crops Res, (1990), 24, p227-240.
[https://doi.org/10.1016/0378-4290(90)90040-i]

- E.P. Columbus, M.J. Fuller, Factors affecting kenaf fiber and core separation. Kenaf properties: Processing and products, Starkville, MS, USA, Mississippi State University, (1999), p83-89.
-
C.G. Cook, M.V. Hickman, C.L. Webber, J.W. Sij, A.W. Scotte, Fungicide treatment effects on kenaf seed germination and stand establishment., Ind. Crops Prod, (1992), 1, p41-45.
[https://doi.org/10.1016/0926-6690(92)90044-v]

-
P.S. Curtis, A. Läuchli, Responses of kenaf to salt stress: Germination and vegetative growth., Crop Sci, (1985), 25, p944-949.
[https://doi.org/10.2135/cropsci1985.0011183x002500060011x]

-
P.S. Curtis, A. Läuchli, The role of leaf area development and photosynthetic capacity in determining growth of kenaf under moderate salt stress., Aust. J. Plant Physiol, (1986), 13, p553-565.
[https://doi.org/10.1071/pp9860553]

-
N.G. Danalatos, S.V. Archontoulis, Growth and biomass productivity of kenaf(Hibiscus cannabinus L.) under different agricultural inputs and management practices in central Greece., Ind. Crops Prod, (2010), 32, p231-240.
[https://doi.org/10.1016/j.indcrop.2010.04.013]

- I.O. Daniel, O.N. Adeniyan, J.A. Adetumbi, M.A. Okelana, S.A. Olakojo, M.O. Ajala, O.A. Aluko, M.A. Adekoya, Hydropriming improved germination and vigour of kenaf(Hibiscus cannabinus L.) seeds., J. Food Agric. Environ, (2012), 10, p760-763.
- P.A. Davision, C.M. Bray, Protein synthesis during osmopriming of leek(Allium porrum L.) seeds., Seed Sci. Res, (1991), 1, p29-35.
- I. Duman, D. Esiyok, Effect of pre-sowing PEG and KH2PO4 treatments on germination emergence and yield of carrot., Turk. J. Agric. For, (1998), 22, p445-449.
-
T.L. Finnerty, J.M. Zajicek, M.A. Hussey, Use of seed priming to bypass stratification requirements of three Aquilegia species., HortScience, (1992), 27, p310-313.
[https://doi.org/10.21273/hortsci.27.4.310]

- L.E. Francois, T.J. Donovan, E.V. Maas, Salt tolerance of kenaf., Advances in new crops, J. Janick, J.E. Simon, Portland, OR, USA, Timber Press, (1990), p300-301.
-
L.E. Francois, T.J. Donovan, E.V. Maas, Yield, vegetative growth, and fiber length of kenaf grown on saline soil., Agron. J, (1992), 84, p592-598.
[https://doi.org/10.2134/agronj1992.00021962008400040010x]

-
S.H. Gurusinghe, Z. Cheng, K.J. Bradford, Cell cycle activity during seed priming is not essential for germination advancement in tomato., J. Exp. Bot, (1999), 50, p101-106.
[https://doi.org/10.1093/jxb/50.330.101]

- A.I. Ismail, M.M. El-Araby, A.Z.A. Hegazi, S.M.A. Moustafa, Optimization of priming benefits in tomato(Lycopersicon esculentum M.) and changes in some osmolytes during the hydration phase., Asian J. Plant Sci, (2005), 4, p691-701.
- M.M. Jahangir, M. Amjad, I. Afzal, Q. Iqbal, A. Nawaz, Lettuce achene invigoration through osmopriming at supraoptimal temperature., Pak. J. Agric. Sci, (2009), 46, p1-6.
- M. Janmohammadi, P.M. Dezfuli, F. Sharifzadeh, Seed invigoration techniques to improve germination and early growth of inbred line of maize under salinity and drought stress., Gen. Appl. Plant Physiol, (2008), 34, p215-226.
- C.W. Jin, A.K. Ghimeray, L. Wang, M.L. Xu, J.P. Piao, D.H. Cho, Far infrared assisted kenaf leaf tea preparation and its effect on phenolic compounds, antioxidant and ACE inhibitory activity., J. Med. Plants Res, (2013), 7, p1121-1128.
- J.S. Kang, I.S. Choi, Leakage of seed reserve nutrient in artificially aged pepper seeds and enhancement of seed vigor by priming., J. Life Sci, (2006), 16, p352-356.
- S.M. Kang, H.G. Kim, W.H. Yang, S.H. Yong, D.J. Park, J.H. Park, E.H. Enukwa, M.S. Choi, Changes in the physicochemical characteristics of sancho oil according to the purification process., Hanguk Yakyong Changmul Hakhoe Chi, (2017), 25, p296-304.
- J.D. Kim, C.H. Kwon, S.H. Chae, S.N. Hur, J.G. Kim, Effect of priming materials and its concentrations on the germination of pasture seed., Journal of Korean Grassland Science, (2006), 26, p277-284.
- K.B. Lee, S.B. Lee, S.W. Hwang, J.H. Jeong, N.H. Baek, The Third new continent, reclaimed land, Suwon, Korea, Rural Development Administration, (2012), p5.
- S.H. Lee, Y. An, S.H. Yoo, S.M. Lee, Changes in early stage vegetation succession as affected desalinization process in Dea-Ho reclaimed land., Korean Journal of Environmental Agriculture, (2000), 19, p364-369.
- X.R. Li, C.Y. Yu, I.S. Kim, Effects of pre-sowing seed treatments on germination and seedling emergence of carrot., J. Agric. Sci, (1999), 10, p10-17.
- E.V. Maas, G.J. Hoffman, Crop salt tolerance-current assessment., J. Irrig. Drain. Div, (1977), 103, p115-134.
- M.B. McDonald, Seed priming., Seed technology and its biological basis, M. Black, J.D. Bewly, Boca Raton, FL, USA, CRC Press LLC, (2000), p287-325.
- H. Nayyar, C.P. Malik, Alleviation of drought stress in pigeonpea with mixtalol seed priming., J. Agric. Sci, (1993), 13, p27-30.
-
Y.Y. Oh, S.H. Lee, Y.J. Kim, J.T. Kim, J.H. Ryu, S. Kim, J. Jin, H.S. Bae, S.H. Lee, Y.D. Kim, H.C. Hong, S.L. Kim, Influence of seed germination by treatment of seed dormancy in Indian Jointvetch(Aeschynomene Indica L.) seed., Journal of Korean Society International Agriculture, (2015), 27, p663-666.
[https://doi.org/10.12719/ksia.2015.27.5.663]

-
S.C. Rao, W.A. Phillips, Effect of seed priming and soil residue on seedling emergence and forage production of Brassicas., J. Sustain. Agric, (1993), 3, p89-98.
[https://doi.org/10.1300/j064v03n02_08]

- E.H. Roberts, Seed storage for genetic conservation., Plant Today, (1989), 2, p12-18.
-
J. Ryu, B.K. Ha, D.S. Kim, J.B. Kim, S.H. Kim, S.Y. Kang, Assessment of growth and seed oil composition of kenaf(Hibiscus cannabinus L.) germplasm., J. Crop Sci. Biotechnol, (2013), 16, p297-302.
[https://doi.org/10.1007/s12892-013-0074-x]

- J. Ryu, S.J. Kwon, J.W. Ahn, B.K. Ha, S.W. Jeong, S.B. Im, J.B. Kim, S.H. Kim, Y.K. Lee, S.Y. Kang, Evaluation of nutritive value and identification of fungi in silage from new kenaf(Hibiscus cannabinus L.) cultivars., Int. J. Agric. Biol, (2016), 18, p1159-1168.
-
F. Shekari, S.H. Mustafavi, A. Abbasi, Sonication of seeds increase germination performance of sesame under low temperature stress., Acta Agric. Slov, (2015), 105, p203-212.
[https://doi.org/10.14720/aas.2015.105.2.03]

-
Y.T. Wang, Using ground kenaf stem core as a major component of container media., J. Am. Soc. Hortic. Sci, (1994), 119, p931-935.
[https://doi.org/10.21273/jashs.119.5.931]

- M. Yaldagard, S.A. Mortazavi, F. Tabatabaie, Influence of ultrasonic stimulation on the germination of barley seed and its alpha-amylase activity., Afr. J. Biotechnol, (2008), 7, p2465-2471.
-
L.S. Yazan, J.B. Foo, S.A.A. Ghafar, K.W. Chan, P.M. Tahir, M. Ismail, Effect of kenaf seed oil from different ways of extraction towards ovarian cancer cells., Food Bioprod. Process, (2011), 89, p328-332.
[https://doi.org/10.1016/j.fbp.2010.10.007]