
Analysis of Chromosome Composition of Gastrodia elata Blume by Fluorescent in situ Hybridization using rDNA and Telomeric Repeat Probes
© The Korean Society of Medicinal Crop Science. All rights reserved.
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License ( http://creativecommons.org/licenses/by-nc/3.0 ) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Gastrodia elata Blume is a saprophytic perennial plant in the Orchidaceae family, because of its agricultural and medicinal effectiveness, researchers focus on its genome and chemical components. However, cytogenetic information based on the chromosome structure and composition to construct chromosomal backbone for genome sequencing research and for the development and breeding of plants is very limited.
We determined the metaphase chromosome composition of the G. elata genome by fluorescence in situ hybridization (FISH) using 5S and 45S rDNAs and telomeric repeat probes. The nuclear genome of G. elata was organized into 2 n = 36, with relatively small (2.71 - 5.50μm)chromosomes that showed gradual decrease in size. Conglutination phenomenon was observed among the metaphase chromosomes, and it was distinguished from that in other plant metaphase chromosome spreads. One pair of signal was detected for each 5S and 45S rDNA in the pericentromeric region and interstitial region on the short arm of chromosomes 10 and 4, respectively, and telomeric DNA signals were detected in the terminal region of most chromosomes.
To our knowledge, this is the first FISH chromosome composition result in G. elata and could be useful in more comprehensive molecular cytogenetic and genomic analyses as well as breeding programs of the medicinal plant G. elata.
Keywords:
Gastrodia elata Blume, Chromosome, 5S rDNA, 45S rDNA, FISH, Telomeric RepeatINTRODUCTION
The genus Gastrodia belongs to the Orchidaceae family, consists of more than 60 species and is mainly distributed in Asia and Oceania (Hsu and Kuo, 2010). It is characterized as a genus of achlorophyllous orchids with succulent tubers and leafless shoot (Suetsugu, 2017). Among the species, G. elata Blume is a saprophytic perennial plant and mainly distributed in Korea, Japan, and the central part of China (Taguchi et al., 1981; Park et al., 2012; Park and Lee, 2013).
G. elata has no roots, leaves, and chlorophylls and is non-photosynthetic. For this reason, G. elata has to grow on rotted wood in symbiosis with a fungus Armillaria mellea and it depends on the secretion of lysozyme from A. mellea to digest plant tissues in order to absorb nutrients (Wang et al., 1999; Park et al., 2012; Park and Lee, 2013).
G. elata is an important medicinal resource and has been used as a traditional medicine for over 1,500 years (Deni, 1995). It is rich in gastordin and other effective ingredients which have high salutary effects (Taguchi et al., 1981). It is also used as anti-convulsant, neurostimulator, anti-aging, sedative, hypnotic, anti-inflammatory, immunostimulant, and antihypertensive alternative drug in oriental medicine (Park et al., 2010; 2012; Park and Lee, 2013). With its medicinal value, less investment, fast production and remarkable economic benefit, cultivation of G. elata are expected to provide many advantages in agriculture (Lan and Xu, 2010).
G. elata has a chromosome number of 2 n = 36 (Aoyama and Yokota, 2011). The conglutination of chromosomes was observed in meiosis metaphase chromosomes, and it occurred between two or more than two pairs of chromosomes (Liang, 1984). In length, the karyotype of metaphase chromosome was reported as a bimodal karyotype, which was asymmetric with small and large without any intermediate size chromosomes (Aoyama and Yokota, 2011).
Most of the publications of G. elata are about the chemical composition and its efficacy. Despite the usefulness of the plant, there is very limited chromosomal study essential for the genome sequencing research and the development and breeding of the plant.
Previous cytogenetic studies in Gastrodia include the chromosome number in G. angusta (Liang, 1984) and the chromosome number and length range in G. confusa, G. elata (Aoyama and Tanaka, 1986), G. pubilabiata (Nakata, 2004), G. javanica Blume (Aoyama and Yokota, 2011), G. gracilis, G. nipponica, and G. flavilabella (Aoyama and Lee, 2012) by the conventional staining method. But until now, there is no fluorescence in situ hybridization (FISH)chromosome information in Gastrodia.
FISH, where fluorescence-labeled probes hybridize to target loci in chromosomal regions (Devi et al., 2005), is a powerful tool for chromosomal studies. FISH techniques made it possible to detect specific DNA sequences useful for chromosome identification (Speicher et al., 1996) and could provide a visible and mappable genomic information at the molecular and chromosomal level.
The genomic DNA of plant species comprises a high range of repetitive DNA sequences which take part in important cellular mechanisms (Han et al., 2008; Singh et al., 2009).
The most prominent repetitive DNA classes are ribosomal RNA genes (rDNAs) which include two major families, the 5S rDNA which has approximately 120-bp long coding and intergenic region (Waminal and Kim, 2012; Zhang et al., 2016), and the 45S rDNA which contains 18S, 5.8S, and 26S coding regions, and the ITS (internal transcribed spacer) and NTS (non-transcribed spacer) non-coding regions. These two classes of rDNAs are highly tandemly repeated in eukaryotic genomes, and the copy number of both 5S and 45S rDNAs range from tens to thousands (Wang and Lemos, 2017). For this reason, 5S and 45S rDNAs are frequently used as primary probes in FISH procedures (Hasterok et al., 2001; Belandres et al., 2015).
In this study, we analyzed FISH chromosome composition using both 5S and 45S rDNAs and the Arabiposis-type telomere repeat sequence (TTTAGGG)n as probes in G. elata, and with the distribution and localization of both rDNA and telomeric signals, relatively detailed chromosomal information of G. elata genome was elucidated.
This FISH chromosomal report of G. elata provides basic chromosome backbone for genome sequence and chromosome organization study which will be useful for more comprehensive molecular cytogenetic and genomic analyses as well as breeding programs of the medicinal plant G. elata.
MATERIALS AND METHODS
1. Plant samples
Meristem tips of the Gastrodia elata protocorms were provided by Forest Biotechnology Division, Forest Genetic Resources Department, National Institute of Forest Science. In brief, seeds of G. elata were harvested after artificial pollination and subsequently germinated by inoculating with the symbiotic fungus, KFRI1212 as previously described (Park et al., 2012; Park and Lee, 2013).
Protocorms were then maintained in the protocorm growth medium (PGM; sucrose 20 g/ℓ, peptone 2 g/ℓ, hyponex powder 2 g/ℓ, agar 8 g/ℓ, pH 4.5) at 24℃ in the dark before meristem tips of protocorms were used for chromosome slide preparation.
2. Slide preparation
Meristems of the protocorms were harvested and pretreated in N2O for 5 hrs at 0.7 Mpa, fixed with Carnoy’s solution (absolute ethanol : glacial acetic acid, 3 : 1 v/v) overnight, and then stored in 70% ethanol for future use.
Chromosome slides were prepared according to Waminal et al. (2011) with some modifications. Briefly, 2㎜ meristem tips were dissected and digested in 1 : 2 (%) ratio of pectolyase : cellulase for 90 min at 37℃. Then the root tips were washed with distilled water. Carnoy’s solution was pipetted into meristematic tissue, squashed, and vortexed for 15 sec. The suspension was then centrifuged at 5,000 rpm for 5 min, and the supernatant was decanted carefully.
The protoplast was re-suspended in acetic acid : ethanol (9 : 1 v/v) solution. The final suspension was spread on a 70℃ pre-warmed glass slide in a humid chamber and air dried at room temperature.
3. Fluorescence in situ hybridization (FISH)
For FISH analysis, the pre-labelled oligoprobes (PLOP) of 5S rDNA, 45S rDNA, and telomeric repeat designed by Waminal et al. (2017) were used.
The 40㎕ hybridization mixture was prepared for each slide following the preparation method described by Waminal and Kim (2015), with some modifications. The mixture included 50% formamide, 10% dextran sulfate, 2xSSC, 50 ng 5S rDNA probe, 25 ng 45S rDNA probe, 50 ng Arabidopsis telomeric repeat probe, and distilled water to make up a value of 40㎕. The hybridization mixture was placed on each slide, and chromosomal DNA was denatured on an 80℃ slide heater for 5 min.
The slides were incubated in a pre-warmed humid chamber at room temperature for 30 min for the probechromosome hybridization step. Post-incubation stringent washes were carried out by washing with 2 × SSC for 10 min at RT, 0.1 × SSC for 25 min at 42℃, and 2 × SSC for 5 min at RT. The slides were dehydrated at RT through an ethanol series (70%, 90%, and 100%) for 3 min each. The slides were dried in the dark. Counterstain with 4’, 6-diamidino-2-phenylindole (DAPI) (Roche 70217321, Hoffmann-La Roche Ltd., Basel, Switzerland) in a ratio 1 : 100 of DAPI (100㎍/㎖ stock) : vectashield (Vector H1000, Vector Laboratories, Burlingame, CA, USA).
Slides were observed under an Olympus BX53 fluorescence microscope (Olympus Co., Tokyo, Japan) with a built-in CCD camera (CoolSNAP, Photometrics, Tucson, AZ, USA), using an oil lens (× 100 magnification).
The most highly constricted and well-spread metaphase chromosomes was analyzed chromosome length was measured using ImageJ, ver. 1.51 k and images were finalized using Adobe Photoshop CS6.
Homologous chromosomes were identified based on their FISH signals, morphological characteristics, and lengths. Chromosome types were classified according to Waminal et al. (2011).
RESULTS AND DISCUSSION
The chromosome complement of Gastrodia elata was 2 n = 36 corroborating with previous results (Liang, 1984; Aoyama and Yokota, 2011; Aoyama and Lee, 2012).
Mitotic metaphase chromosomes of G. elata stained with DAPI exhibited conglutination (Fig. 1), similar to what Liang (1984) had reported. In his report, chromosome conglutination was observed in meiotic metaphase chromosomes, and the conglutination occurred among several chromosomes. Gardener et al. (2006) mentioned that once the telomeres of a chromosome were broken, the new ends of the chromosome could be sticky and tend to fuse with other chromosomes.
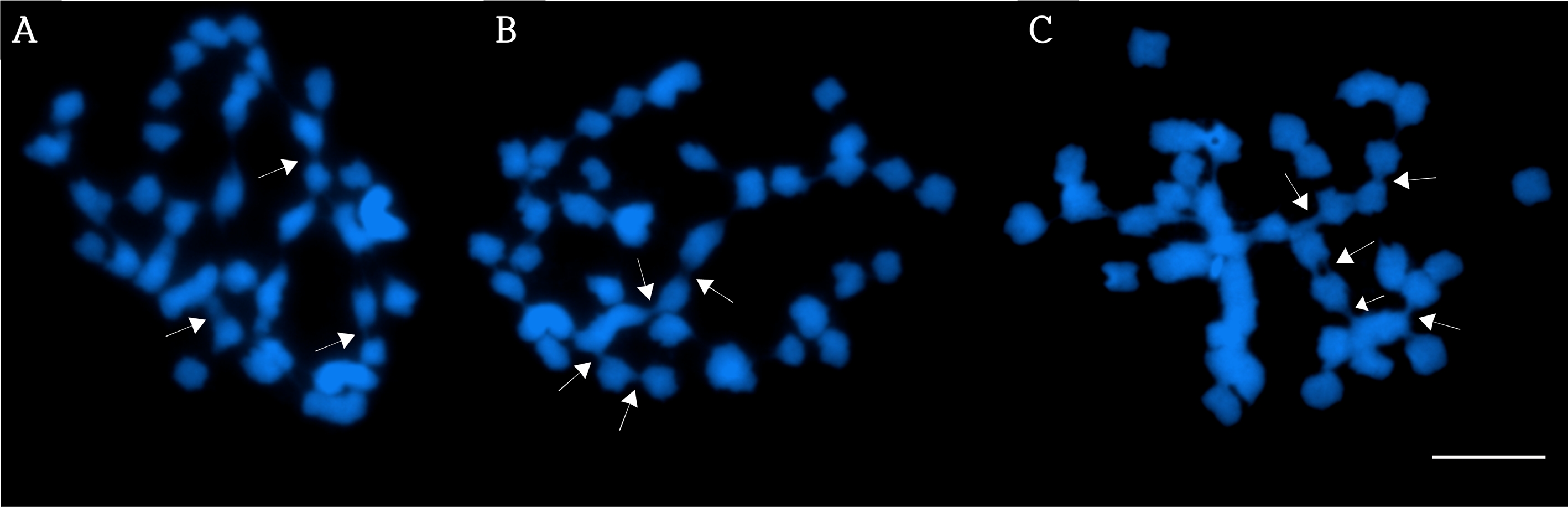
Three mitotic metaphase spreads.All the spreads A, B and C revealed the sticky ends in several chromosomes. The white arrows indicate some examples of the sticky ends. Bar = 10 ㎛.
The reason for the conglutination in this study (Fig. 1) could be the result of broken telomeres but more study needs to explain the phenomenon more exactly.
Karyotypic analysis through conventional staining method provides basic chromosomal information on the genome with a number, size, type, nucleolar organizing region (NOR) etc. (Lim et al., 2001).
Chromosomal banding, fluorescence in situ hybridization, and spectral karyotyping have been developed to show more detailed chromosomal information (Hwang et al., 2010) which have contributed to identifying each species, tracing phylogenetic relationship, and breeding species.
Much research has been reported on the pharmaceutical effect of G. elata and gastrodin is known as the main active ingredient (Zhan et al., 2016) and so the future development and breeding program is an interest.
This chromosome study provides basic cytogenetic information useful for the crop breeding but very limited chromosomal research has been reported until now in spite of its medicinal importance.
FISH technology is an important non-radioactive in situ hybridization. If the target DNA on the detected chromosome is homologous and complementary to the used nucleic acid probe, by denaturation, annealing, and renaturation, hybrid of the target and nucleic acid probes could be formed (Yang et al., 2011; Zhang et al., 2016).
The primarily used DNA markers 5S and 45S ribosomal DNAs (rDNAs) (Belandres et al., 2015) are commonly and abundantly existed as tandem repeats in plants (Zhang et al., 2016). In general, these rDNAs are localized at one or more than one sites at different chromosomes (Shuxin et al., 2007), and so have been used as primary FISH probes for molecular cytogenetic study (Hasterok et al., 2001).
FISH karyotyping is a useful method to provide detailed chromosomal information of a genome (Waminal and Kim, 2015). FISH karyotyping using multiple repetitive DNA probes could explain the intergenic translocation and interspecific anagenesis (Jiang and Gill, 2006).
In our study, FISH chromosome composition was elucidated in G. elata using three kinds of repetitive DNA probes of 5S rDNA, 45S rDNA, and telomeric repeat DNA. One pair of 5S (Fig. 2A) and one pair of 45S rDNA (Fig. 2B) signals were detected in the pericentromeric region of chromosome 10 and interstitial region on the short arm of chromosome 4, respectively (Fig. 3).
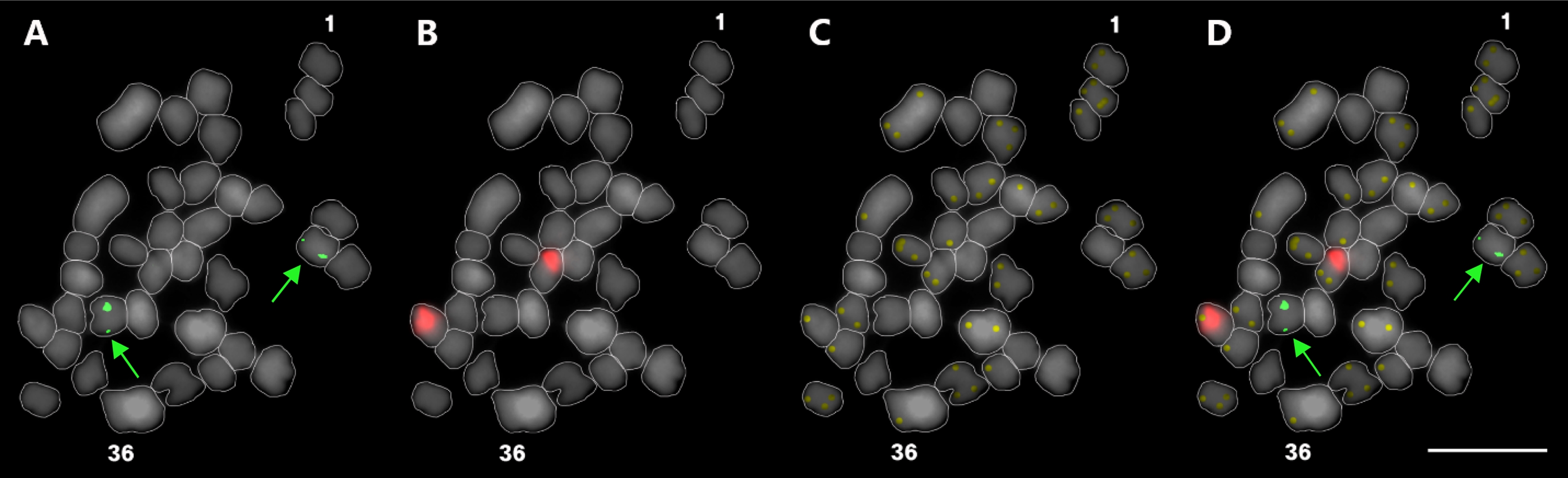
Triple-color FISH metaphase chromosome spread of G. elata.5S rDNA (A), 45S rDNA (B), and telomere repeat signals (C) are shown as red, green, and yellow color, respectively. The merged image is shown in panel D. The total chromosome numbers are indicated with the number 1 as the first and 36 as the last in each panel. The green arrows in A and D indicate the 5S rDNA signals. Bar = 10㎛.
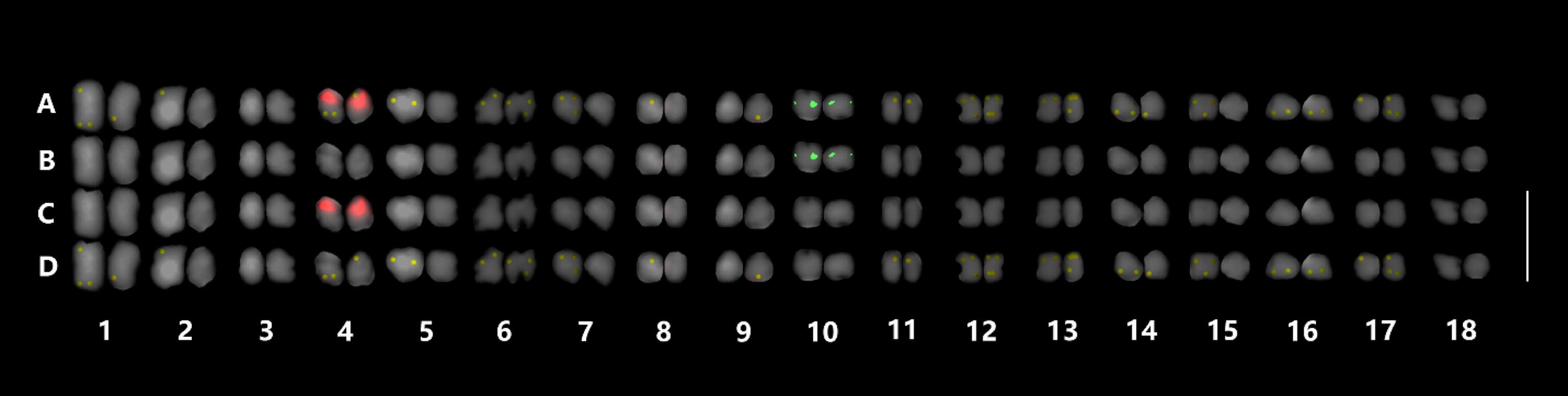
Triple-color FISH karyogram of G. elata.FISH metaphase chromosomes of G. elata were arranged in a decreasing order according to the length and morphology of each chromosome. Merged (A), 5S rDNA (green, B), 45S rDNA (red, C), and telomeric signals (yellow, D) are shown on metaphase chromosomes. Bar = 10㎛.
5S rDNA signals were considerably weaker compared with 45S rDNA signals. Telomeric DNA signals were also very weak and were detected in the telomeric region of most chromosomes (Fig. 2C, Fig. 3). This is the first FISH karyotyping report in G. elata.
According to the length and morphology of each chromosome measured by ImageJ ver. 1.51 k and edited by Adobe Photoshop CS6, we arranged all the chromosomes in decreasing order (Waminal and Kim, 2012), and then paired them based on chromosome morphology and rDNA probe signals (Fig. 3).
Aoyama and Yokota (2011) and Aoyama and Lee (2012) reported that G. elata has morphological similarity with G. javanica and both of them have a bimodal karyotype with a chromosome number of 2 n = 36. But in this result (Fig. 3), a gradual decreasing in chromosome length was revealed.
The most constricted and well-spread metaphase chromosome length was in the range of 2.71 - 5.50㎛ and a total length of 63.19㎛ (Table 1), which was different from Aoyama and Lee’s (2012) report of 1.00 - 3.00㎛ and a total length of 42.70㎛. In general, the most highly coiled metaphase chromosomes in the same species show similar length range, however, there can be the difference in some species.
Li (1985) mentioned that in artificially controlled selfing, the offspring tended to have smaller chromosomes, on the other hand, the chromosomes of offspring restored to normal size when the outcrosses resumed. This variation was caused by a recessive mutation.
In addition, healthy plant, main roots of a plant, and one-week young seedlings had a tendency to have bigger chromosomes as well (Li, 1985). In this study, the root samples were obtained from artificially cultured protocorm, we may assume that there might happen changes in chromosome size.
The chromosome type is usually determined by arm ratio by centromeric position. In this study, we could not discriminate the exact centromeric positions on the metaphase chromosomes by DAPI and conventional staining method which is quite unusual for other plant metaphase chromosomes we have analyzed so far.
Conglutination of metaphase chromosomes is also a rare case in plant metaphase chromosomes. Considering no roots, leaves, and chlorophylls in spite of being classified as a medicinal plant, those chromosomal differences might be natural in G. elata.
Further FISH researches are needed to discriminate centromere and accordingly to analyze arm ratio and determine each chromosome type for refined karyotype of G. elata. This might be possible by FISH using centromere-specific DNA probes which can be mined from genome sequencing data by way of repeat exploring algorithms. Further molecular cytogenetic studies using newly developed chromosome markers will contribute to elucidating the chromosomal organization of its genome.
References
-
Aoyama, M., Lee, Y.I., (2012), Cytological studies on three species ofGastrodia, myco-heterotrophic orchids., Chromosome Botany, 7, p113-116.
[https://doi.org/10.3199/iscb.7.113]

- Aoyama, M., Tanaka, R., (1986), Karyomorphological studies inGastrodiaelata and G. confusa., La Kromosomo, 42, p1336-1340.
- Aoyama, M., Yokota, M., (2011), Chromosome number ofGastrodiajavanica(Blume) Lindl., a myco-heterotrophic orchid., Chromosome Sci, 14, p39-40.
-
Belandres, H.R., Waminal, N.E., Hwang, Y.J., Park, B.S., Lee, S.S., Huh, J.H., Kim, H.H., (2015), FISH karyotype and GISH meiotic pairing analyses of a stable intergeneric hybrid xBrassicoraphanus line BB#5., Weonye Gwahag Gisulji, 33, p83-92.
[https://doi.org/10.7235/hort.2015.14151]

- Deni, B., (1995), Encyclopedia of herbs and their uses, Dorling Kindersley, Londos, England, p154-168.
- Devi, J., Ko, J.M., Seo, B.B., (2005), FISH and GISH: Modern cytogenetic techniques., Indian J. Biotechnol, 4, p307-315.
- Gardner, E.J., Simmons, M.J., Snustad, D.P., (2006), Principles of genetics, 8th ed., John Wiley and Sons, Hoboken, NJ, USA, p229-231.
- Han, Y.H., Zhang, Z.H., Liu, J.H., Lu, J.Y., Huang, S.W., Jin, W.W., (2008), Distribution of the tandem repeat sequences and karyotyping in cucumber(Cucumis sativus L.) by fluorescence in situ hybridization., Cytogenet. Genome Res, 122, p80-88.
-
Hasterok, R., Jenkins, G., Langdon, T., Jones, R.N., Maluszynska, J., (2001), Ribosomal DNA is an effective marker of Brassica chromosomes., Theor. Appl. Genet, 103, p486-490.
[https://doi.org/10.1007/s001220100653]

- Hsu, T.C., Kuo, C.M., (2010), Supplements to the orchid flora of Taiwan(IV): Four additions to the genusGastrodia., Taiwania, 55, p243-248.
- Hwang, Y.J., Lee, S.N., Song, K.A., Ryu, K.B., Ryu, K.H., Kim, H.H., (2010), Karyotype analyses of genetically modified(GM) and Non-GM hot peppers by conventional staining and FISH method., Hortic. Environ. Biotechnol, 51, p525-530.
-
Jiang, J., Gill, B.S., (2006), Current status and the future of fluorescence in situ hybridization(FISH) in plant genome research., Genome, 49, p1057-1068.
[https://doi.org/10.1139/g06-076]

- Lan, J., Xu, J.T., (2010), Technique for cultivation ofGastrodiaelata BaiwenBaida, China Agriculture Press, Beijing, China, p101.
- Li, M., (1985), Variation and evolution of plant chromosome size., Bulletin of Biology, 5, p14-16.
- Liang, H., (1984), Investigation of the chromosome number ofGastrodiaangusta., Acta Botanica Yunnanica, 6, p311-312.
-
Lim, K.B., Wennekes, J., de Jong, J.H., Jacobsen, E., van Tuyl, J.M., (2001), Karyotype analysis of Lilium longiflorum and Lilium rubellum by chromosome banding and fluorescence in situ hybridization., Genome, 44, p911-918.
[https://doi.org/10.1139/g01-066]

- Nakata, M., (2004), Chromosome number ofGastrodiapubilabiata (Orchidaceae)., Bulletin of the Botanical Garden of Toyama, 9, p74.
-
Park, E.J., Lee, W.Y., (2013), In vitro symbiotic germination of myco-heterotrophicGastrodiaelata by Mycena species., Plant Biotechnol. Rep, 7, p185-191.
[https://doi.org/10.1007/s11816-012-0248-x]

-
Park, E.J., Lee, W.Y., Ahn, J.K., (2012), In vitro propagation of myco-heterotrophicGastrodiaelata., Hortic. Environ. Biotechnol, 53, p415-420.
[https://doi.org/10.1007/s13580-012-0046-y]

- Park, E.J., Lee, W.Y., Kim, S.T., Ahn, J.K., Bae, E.K., (2010), Ergothioneine accumulation in a medicinal plantGastrodiaelata., J. Med. Plants Res, 4, p1141-1147.
- Shuxin, X., Shuxing, S., Jianjun, Z., (2007), Location of 25S rDNA and 5S rDNA in Chinese cabbage-pe-tsai metaphase chromosome., Scientia Agricultura Sinica, 40, p782-787.
-
Singh, M., Kumar, R., Nagpure, N.S., Kushwaha, B., Mani, I., Chauhan, U.K., Lakra, W.S., (2009), Population distribution of 45S and 5S rDNA in golden mahseer, Tor putitora: Populationspecific FISH marker., J. Genet, 88, p315-320.
[https://doi.org/10.1007/s12041-009-0045-7]

-
Speicher, M.R., Ballard, S.G., Ward, D.C., (1996), Karyotyping human chromosomes by combinatorial multi-fluor FISH., Nat. Genet, 12, p368-375.
[https://doi.org/10.1038/ng0496-368]

- Suetsugu, K., (2017), Two new species ofGastrodia(Gastrodieae, Epidendroideae, Orchidaceae) from Okinawa Island, Ryukyu Island, Japan., Phytotaxa, 302, p251-258.
-
Taguchi, H., Yosioka, I., Yamasaki, K., Kim, I.H., (1981), Studies on the constituents ofGastrodiaelata Blume., Chem. Pharm. Bull. (Tokyo), 29, p55-62.
[https://doi.org/10.1248/cpb.29.55]

-
Waminal, N.E., Kim, H.H., (2012), Dual-color FISH karyotype and rDNA distribution analyses on four Cucurbitaceae species., Hortic. Environ. Biotechnol, 53, p49-56.
[https://doi.org/10.1007/s13580-012-0105-4]

-
Waminal, N.E., Kim, H.H., (2015), FISH karyotype analysis of four wild Cucurbitaceae species using 5S and 45S rDNA probes and the emergence of new polyploids in Trichosanthes kirilowii maxim., Korean Journal of Horticultural Science and Technology, 33, p869-876.
[https://doi.org/10.7235/hort.2015.15101]

-
Waminal, N.E., Choi, H.I., Kim, N.H., Jang, W., Lee, J., Park, J.Y., Kim, H.H., Yang, T.J., (2017), A refined Panax ginseng karyotype based on an ultra-high copy 167-bp tandem repeat and ribosomal DNAs., J. Ginseng Res, 41, p469-476.
[https://doi.org/10.1016/j.jgr.2016.08.002]

-
Waminal, N.E., Kim, N.S., Kim, H.H., (2011), Dual-color FISH karyotype analyses using rDNAs in three Cucurbitaceae species., Genes Genomics, 33, p521-548.
[https://doi.org/10.1007/s13258-011-0046-9]

-
Wang, M, Lemos, B, (2017), Ribosomal DNA copy number amplification and loss in human cancers is linked to tumor genetic context, nucleolus activity, and proliferation., PLoS Genetics, 13, pe1006994.
[https://doi.org/10.1371/journal.pgen.1006994]

- Wang, X.C., Diaz, W.A., Bauw, G., Xu, Q., Montagu, M.W., Chen, Z.L., Dillen, W., (1999), Molecular cloning of GAFP-1, an antifungal protein fromGastrodiaelata., Acta Bot. Sin, 41, p1041-1045.
-
Yang, K., Zhang, H., Converse, R., Wang, Y., Rong, X., Wu, Z., Luo, B., Xue, L., Jian, L., Zhu, L., Wang, X., (2011), Fluorescence in situ hybridization on plant extended chromatin DNA fibers for single-copy and repetitive DNA sequences., Plant Cell Rep, 30, p1779-1786.
[https://doi.org/10.1007/s00299-011-1086-y]

- Zhan, H.D., Zhou, H.Y., Sui, Y.P., Du, X.L., Wang, W.H., Dai, L., Sui, F., Huo, H.R., Jiang, T.L., (2016), The rhizome ofGastrodiaelata Blume-an ethnopharmacological review., J. Ethnopharmacol, 189, p361-385.
-
Zhang, Z.T., Yang, S.Q., Li, Z.A., Zhang, Y.X., Wang, Y.Z., Cheng, C.Y., Li, J., Chen, J.F., Lou, Q.F., (2016), Comparative chromosomal localization of 45S and 5S rDNAs and implications for genome evolution in Cucumis., Genome, 59, p449-457.
[https://doi.org/10.1139/gen-2015-0207]

