Enhancement of Solubility and Nanonization of Phenolic Compound in Extrudate from Angelica gigas Nakai by Hot Melt Extrusion using Surfactant
© The Korean Society of Medicinal Crop Science. All rights reserved.
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License ( http://creativecommons.org/licenses/by-nc/3.0 ) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The root of Angelica gigas Nakai is used as a traditional herbal medicine in Korea for the treatment of many diseases. However, the poor water solubility of the active components in A. gigas Nakai is a major obstacle to its bioavailability.
This work aimed at enhancing the solubility of the active compounds of A. gigas Nakai by a chemical (using a surfactant) and physical (hot melt extrusion, HME) crosslinking method. Fourier transform infrared spectroscopy revealed multiple peaks in the case of the extrudate solids, attributable to new functional groups including carboxylic acid, alkynes, and benzene derivatives. Differential scanning calorimetry analysis showed that the extrudate soilid had a lower glass transition temperature (Tg) and enthalpy (ΔH) (Tg : 43°C, ΔH : < 6 J/g) as compared to the non-extrudate (Tg : 68.5°C, ΔH : 123.2) formulations. X-ray powder diffraction analysis revealed the amorphization of crystalline materials in the extrudate solid. In addition, enhanced solubility (53%), nanonization (403 ㎚), and a higher amount of extracted phenolic compounds were achieved in the extrudate solid than in the non-extrudate (solubility : 36%, nanonization : 1,499 ㎚) formulation. Among the different extrudates, acetic acid and span 80mediated formulations showed superior extractions efficiency.
HME successfully enhanced the production of amorphous nano dispersions of phenolic compound including decursin from extrudate solid formulations.
Keywords:
Angelica gigas Nakai, Hot Melt Extrusion, Nano-solid Dispersion, Size Reduction, SolubilityINTRODUCTION
The root of Angelica gigas Nakai (AGR, Angelica gigantis radix), known as Korean angelica, AGR is a herbal medicine for the treatment of various circulatory disorders with female afflictions such as dysmenorrhea, amenorrhea, menopause, abdominal pain, migraine and arthritis (Kim et al., 2009). AGR has several pharmacological properties that make it useful in the treatment of menopausal syndromes, anemia, abdominal pain, inhibition of breast cancer, and amenorrhea (Lee et al., 2003; Ma et al., 2009; Nam et al., 2018) with several coumarin derivates including decursin decursinol, decursinol angelate, nodakenin, nodakenetin and umbelliferone (Kang et al., 2001; Yan et al., 2004).
Generally, methanol and tetrahydrofuran (THF) have been used to successfully extract and quantify lipophilic compounds, but these solvents cannot be used for humans. For the efficient extraction of pharmacologically active components in medicinal plants, ethanol have generally been used as extraction solvents can be the most suitable solvent for effective extraction of lipophilic bioactive compound (Bae et al., 2011). Traditionally, hot water extraction is used to extract bioactive components from medicinal plant such as AGR. However, that method seems to be inappropriate for extracting poorly watersoluble components from AGR (Choi et al., 2012). Several major components of AGR, such as decursin and decursinol angelate are reported as poorly water-soluble, thus improving the aqueous solubility of those active components is necessary.
In addition, a high level of intermolecular covalent bonds in the crystal lattice, the diverse range of structural components, the high bonding capacity within the molecules, and the large molecular weight of the endproduct make it less functional (Khoddami et al., 2013).
There are reports using different processes to enhance the solubility of pharmaceutical and plant food compounds (Sebestyen, 1974). Among these, the hot melt extrusion (HME) process, based on particle size reduction, has gained much attention as a means to improve the solubility of poorly water-soluble compounds (Maniruzzaman et al., 2013; Lee et al., 2017a).
Mechanical disruption during the extrusion process results in a number of changes to the physicochemical characteristics of raw materials, such as enhanced solubility, reduced particle size, loss of water holding capacity, and softening of the product texture (Harper, 1992). During the process, fiber bundles experience compression and high shear, resulting in defibration and the formation of an amorphous mass (Moralse and McConville, 2011; Jurišić et al., 2015).
In addition, research suggests that acid solutions enhance the ionization of the active compound by rupturing the cell wall, thus facilitating the extraction (Vichapong et al., 2010). Moreover, a lower pH is associated with greater stability of flavonoids from plant materials (Hagi and Hatami, 2010; Davidov-Pardo et al., 2011).
Solubility can also be enhanced by a chemical process in which the active compounds are incorporated into a surfactant colloidal micelle without adversely affecting any food-quality attributes (McClements and Xiao, 2012).
Surfactants are surface-active agents composed of a complex mixture of various kinds of molecules possessing strong affinities to polar and nonpolar substances (Hasenhuettl and Harel, 2008). Surfactants have an excellent capacity to form micro-emulsions by lowering the interfacial tension and reducing the laplace pressure (Morsy, 2014). One of the most important properties of surfactants is their ability to enhance the aqueous solubility of poorly soluble compounds and to control the stability and rheology of a particular food composition (Rosen, 2004; Kralova and Sjőblom, 2009).
Amorphous solid dispersion is an important approach to improve the solubility of poorly water-soluble compounds (Baird and Taylor, 2012). Therefore, a dynamic approach was taken to develop an amorphous solid nano dispersion of the active compound from AGR by a chemical (viz. surfactant) and physical (hot melt extrusion) crosslinking (CPC) method using HME.
We assume that this method would speed up the amorphization, enhance the solubility and increase the amount of extracted phenolic compounds from extrudate formulations of AGR.
MATERIALS AND METHODS
1. Chemical and reagents
Acetic acid (1 M), citric acid, tween 80 (hydrophiliclipophilic balance, HLB : 15.0), span 80 (HLB : 4.3), phenolic reagent (Folin Ciocalteu, 2 N), sodium bicarbonate (Na2CO3), aluminum nitrate (AlNO3)3, potassium acetate (CH3CO2K), DPPH (2,2-diphenyl-1picrylhydrazyl), and acetic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals used were of analytical grade and purchased from Merck (Darmstadt, Germany). Deionized, distilled water (EC value < 0.3 μS·㎝−1) was used for sample preparation.
2. Preparation of ultrafine powder of AGR (UFP-AGR)
Coarse powder was prepared from freeze dried AGR. AGR samples were
milled into coarse powder by a pin crusher (JIC-P10-2; Myungsung Machine, Seoul, Korea) equipped with a 30- mesh sieve.
The milled powder was fractionated using a sieve shaker (CG-213, Ro-Top, Hankook Cosmetics Manufacturing Co., Ltd., Seoul, Korea) equipped with a series of sieves (F 20㎝). The powder was passed through 300㎛ mesh size sieves, and unpassed particles were grinded again with the pin crusher. Those powders were then stored at 25℃ before ultrafine milling.
The coarse powders were pulverized and classified by a low temperature turbo mill (HKP-05; Korea Energy Technology Co., Ltd., Seoul, Korea). The temperature of the mill chamber was kept at −18℃. The ultrafine powder of AGR (UFP-AGR) was stored in a desiccator before use.
3. Preparation of chemical formulation
Ethoxylated alcohols are the most common nonionic surfactants. These alcohols contain a wide-ranging degree of ethoxylation, where ethylene oxide is added to fatty acids to make them more water-soluble. They are considered “amphiphiles”, with a lipophilic hydrocarbon tail group and water loving ethoxylated alcohol group (Holmberg et al., 2003). Examples of non-ionic surfactants include sugar esters, such as sorbitan monooleate (span 80). Polyoxyethylene sorbitan monooleate (tween 80) appear safe and acceptable for oral and parenteral use (Lawrence and Rees, 2012).
Acetic acid (100 mM) was prepared in aqueous medium, two types of surfactants, namely, the hydrophilic tween 80 and the lipophilic span 80, were used for chemical fourmulation.
Five grams of each surfactant was added to 100㎖ of distilled water and 100㎖ of 100 mM acetic acid aqueous solution to prepare (5%) the surfactant emulsion and the acid-surfactant emulsion, respectively.
The succeeding steps were performed on both types of emulsions: the solution was homogenized using a digital homogenizer (PRO25D, Proscientific Inc., Oxford, CT, USA) at 10,000 rpm for 5 minutes. One hundred milliliters of emulsion was added to 200 g of UFP-AGR and mixed well using an electric blender to prepare the chemical formulations.
4. Preparation of extrudate formulation and HME configuration
Hot-melting extrusion technique has been used to prepare solid formulations (i.e. solid dispersion) of poorly water-soluble components (Wilson et al., 2012).
The extrudate solid formulation of UFP-AGR was developed using an STS-25HS twin-screw HME (Hankook E.M. Ltd., Pyeongtaek, Korea). The extruder was equipped with a round-shaped die (1㎜) and was operated at a feeding rate of 40 g/min, 150 rpm with high shear. The temperature profile from the feeding zone to die was 80/ 100/100/80/70℃. The UFP-AGR extrudate solid formuation (UFP-AGR-ESF) was dried in an oven at 50℃ and then ground for further analysis.
5. Particle size analysis
UFP-AGR and UFP-AGR-ESF (0.5 g) was suspended in 50 ㎖ of distilled water. The supernatant was separated by centrifugation at 3,000 rpm for 10 min. The particle size of the supernatant was studied using a light-scattering spectrophotometer (ELS-Z1000; Otsuka Electronics Co., Ltd., Tokyo, Japan) with three replications.
6. Solubility measurements
Water solubility was determined according to the previously reported method (Piao et al., 2015). One gram of UFP-AGR and UFP-AGR-ESF powders were suspended in 50㎖ of DW at room temperature, gently stirred for 1 h, and then centrifuged at 3,000 rpm for 10 mins. The supernatant was decanted into an evaporating dish of known weight. Water solubility was calculated by the formula described by Piao et al. (2015).
7. Evaluation of amorpous solid dispersion by FT-IR, DSC, and XRPD
Fourier transform infrared spectroscopy (FT-IR) can be used to detect the variations in vibrational energy between amorphous and crystalline states. Also, differential scanning calorimetry (DSC) is probably the most versatile and widely used technique in the characterization of amorphous formulations including quantifi cation of crystallinity (Williams et al., 2012).
XRPD is typically a nondestructive test and, is highly useful for determining differences in crystal structure, core compounds-excipient interactions, and identifying amorphous systems (Williams et al., 2012).
FT-IR spectra of UFP-AGR-ESF were recorded on a Perkin-Elmer Model 1600 apparatus (Norwalk, CT, USA) using KBr stressed disks in the range of 4,000 - 400·㎝−1. Ten milligrams of each sample was positioned in contact with the attenuated total reflectance (ATR) plate.
All spectra were subtracted against a background of air spectra. After every scan, a new reference of air background spectra was taken. The ATR plate was carefully cleaned by scrubbing with 70% isopropyl alcohol twice followed by drying with soft tissue before being filled in with the next sample, making it possible to dry the ATR plate.
The DSC curves were obtained on a calorimeter (DSC Q2000, TA Instruments, New Castle, DE, USA) using aluminum crucibles with approximately 2.0 ± 0.1㎎ of samples under a nitrogen atmosphere, at a flow of 50㎖·min−1.
Rising temperature experiments were conducted at the temperature range of 20℃ to 250℃ with a heating rate of 10℃·min-1. Indium (melting point, 156.6℃) was used as the standard for equipment calibration. Data were analyzed using the software (Universal Analysis 2000, TA Instruments, New Castle, DE, USA).
The XRPD analysis were carried out in an X'Pert PRO XRD diffractometer (PANalytical B.V., Almelo, Netherlands) that scanned from 10 to 55 (2 min−1) on the 2 h scale and with CuK α1 radiation. The equipment was operated at 40.0 kV and 30.0 mA. The data were analyzed using the Origin® version 8.1 software (OriginLab, Northampton, MA, USA).
8. Extraction of formulation
One gram of UFP-AGR and UFP-AGR-ESF were added to 100㎖ of distilled deionized water. The sample was shaken at 150 rpm, 25℃, using a shaking incubator (SI- 900RF, JEIO TECH, Seoul, Korea) for 1 h.
The sample was filtered through a 125㎜ filter paper (Advantech 5B, Toyo Roshi Kaisha, Tokyo, Japan), and then the extract was collected and stored in the refrigerator at −20℃ for further analysis.
9. Determination of total phenolic contents (TP)
The total phenolic contents were determined by the Folin-Ciocalteu assay (Singleton and Rossi, 1965).
UFP-AGR and UFP-AGR-ESF extract sample aliquot of 200㎕ was added to a test tube containing 200㎕ of phenol reagent (1 N). The volume was increased by adding 1.8㎖ of distilled deionized water. The solution was allowed to react for 3 min. To continue the reaction, 400㎕ of Na2CO3 (10% in water v/v) was added and vortexed. The final volume of 4㎖ was adjusted by adding 1.4㎖ of distilled water. The prepared sample was then incubated for 1 hour at room temperature.
The absorbance was measured at 725㎚ using a spectrophotometer (UV-1800 240 V, Shimadzu, Kyoto, Japan). The TP was expressed as gallic acid equivalents (GAE) on a dry weight basis.
10. Determination of total flavonoid content
The total flavonoid content (TF) was determined according to Ghimeray et al. (2014).
Briefly, a 0.5㎖ aliquot of the UFP-AGR and UFPAGR- ESF extract sample (1㎎/㎖) was mixed with 0.1㎖ of 10% aluminum nitrate and 0.1㎖ of potassium acetate (1 M). To this mixture, 3.3㎖ of distilled water was added to make the total volume 4㎖. The mixture was vortexed and incubated for 40 mins.
The total flavonoids were measured using a spectrophotometer (UV-1800 240 V, Shimadzu, Kyoto, Japan) at 415㎚. The TF was expressed as ㎍/g coumarin equivalents on a dry weight basis.
11. DPPH free radical scavenging activity
The antioxidant activity was determined on the basis of the scavenging activity of the stable 2,2-diphenyl-1picryl hydrazyl (DPPH) free radical according to methods described by Braca et al. (2003).
One milliliter of UFP-AGR and UFP-AGR-ESF extract sample was added to 3㎖ of DPPH (Sigma-Aldrich Co., St. Louis, MO, USA). The mixture was shaken vigorously and left to stand at room temperature in the dark for 30 mins. The absorbance was measured at 517㎚ using a spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan). The percent inhibition activities of the sample were calculated against a blank sample using the following equation: inhibition (%) = (blank sample-extract sample / blank sample) × 100.
12. Statistical analysis
All data were expressed as means ± SD of triplicate measurements. The obtained results were compared among the different surfactants concentration and types using a paired t-test in order to observe the significant differences at the level of 5%. The paired t-test between mean values was analyzed by MINITAB version 16.0 (Minitab Inc., State College, PA, USA).
RESULTS AND DISCUSSION
1. Particle size reduction and solubility enhancement of extrudate solid formuation of Angelica gigantis radix ultrafine powder with chemical formulation.
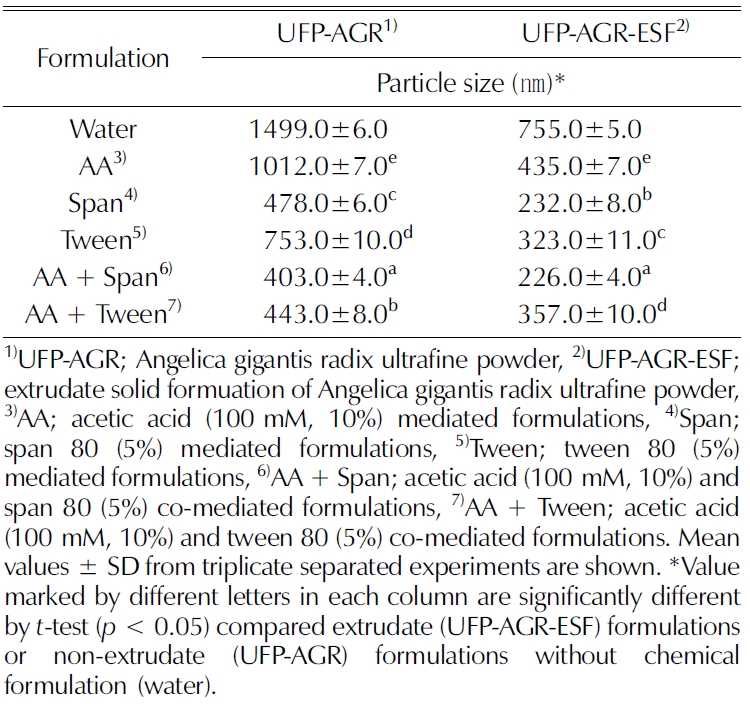
Table 1 shows that nano-sized particles were achieved in the extrudate formulation. The particle size of the nonextrudate sample (UFP-AGR) was recorded at 1499.0 ± 22.0 ㎚, whereas in the extrudate sample (UFP-AGR-ESF), the particle size was 755.0 ± 15.0㎚. Among the formulations, the smallest particle size (226.0 ± 12.0㎚) was achieved in the AA+ span-mediated extrudate formulation.

Particle size analysis of non-extrudate and extrudate of Angelica gigantis radix ultrafine powder by different chemical formulation.
HME is the most suitable process to enhance the amorphization of crystal materials by reducing particle size to enhance solubility (Maniruzzaman et al., 2013; Lee et al., 2017b). The particle size reduction stragety results in increased surface area, decreased diffusional distance, and increased dissolution rates (Hu et al., 2004; Repka et al., 2007; Merisko-Liversidge and Liversidge, 2008).
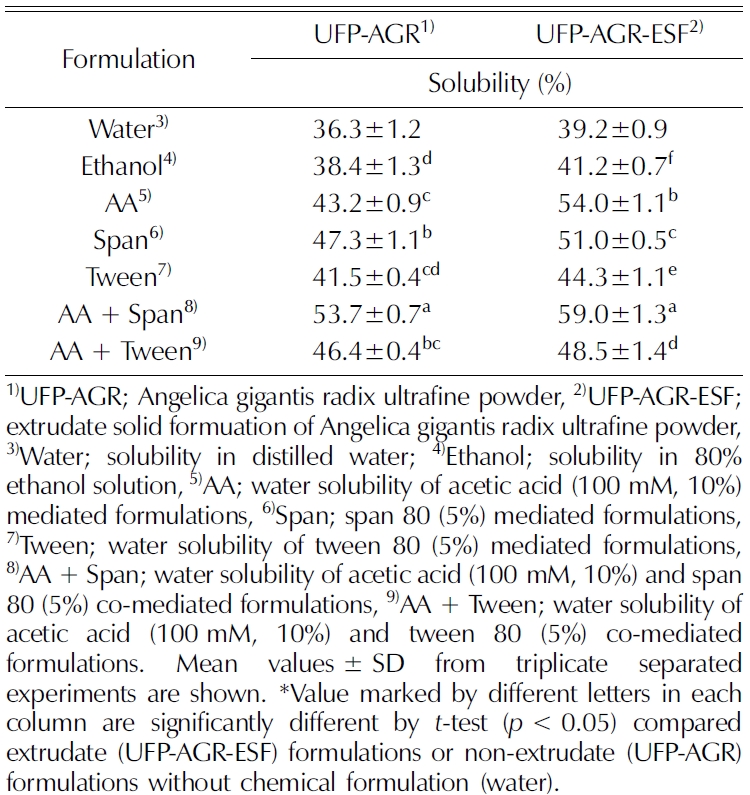
According to Ansel (1985), the surfactant, also called emulsifier plays an important role in emulsion formation by reducing the interfacial tension. They also reported that the surfactants mixture increased the solubility of the bioactive compound in the emulsion. The results in Table 2 show that water solubility was improved in acetic acid and span 80 co-mediated formulations (59%, 53%) compared to control without chemical formulation (39%, 36%) in extrudate (UFP-AGR-ESF) and non-extrudate (UFP-AGR) formulations, respectively.

Water solubility analysis of non-extrudate and extrudate of Angelica gigantis radix ultrafine powder by different chemical formulation.
According to the Noyes-Whitney equation, particle size has a direct effect on the dissolution rate. The reduction of particle size increases the diffusional coefficient and nanonization. In addition, an acidic solution (H+) increases the concentration gradient as well as enhances the dissolution rate (Fox, 2014). Amorphous nanoparticles exhibit very high saturation solubility compared to the crystalline form (Agrawal et al., 2004; Murdande et al., 2010). The HME process tends to make more channels to enhance the permeability and penetration of water into the core of the material’s matrix (Piao et al., 2015).
2. FT-IR, DSC and XRD analysis of extrudate solid formuation of Angelica gigantis radix ultrafine powder with chemical formulation.
FT-IR spectroscopy, used to investigate the possible chemical compounds in the extrudate sample (UFP-AGRESF), is the most suitable technique among the nondestructive spectroscopic methods, since it can detect a range of functional groups and is sensitive to changes in molecular structure (Cocchi et al., 2004; Tita et al., 2011).
The main differences found when comparing the spectra are presented in Fig. 1, which shows that the splitting peak found between 1,700 - 3,500·㎝−1 is absent in the non-extrudate sample (UFP-AGR). However, the extrudate sample (UFP-AGR-ESF) shows a multiple splitting peak in the 1,700 - 3,500·㎝−1 wavelengths, which represents a new functional group.
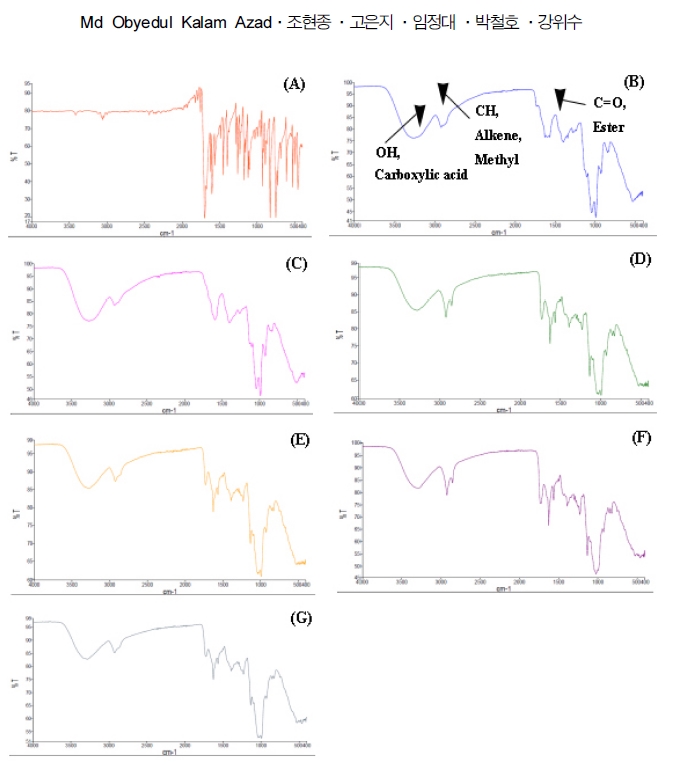
The FT-IR analysis of extrudate solid formuation (UFP-AGR-ESF) by different chemical formulation.(A); non-extrudate, Angelica gigantis radix ultrafine powder (UFP-AGR), (B); extrudate solid formuation of Angelica gigantis radix ultrafine powder (UFP-AGR-ESF) without chemical formulation, (C); UFP-AGR-ESF with acetic acid (100 mM, 10%) mediated formulations, (D); UFP-AGR-ESF with span 80 (5%) mediated formulations, (E); UFP-AGR-ESF with tween 80 (5%) mediated formulations, (F); UFP-AGR-ESF with acetic acid (100 mM, 10%) and span 80 (5%) co-mediated formulations, (G); UFP-AGR-ESF with acetic acid (100 mM, 10%) and tween 80 (5%) co-mediated formulations. Asterisk indicate functional group.
In the extrudate sample (UFP-AGR-ESF), there is a strong peak in wavelength ranges of 2,800 - 3,000·㎝−1, which correspond to alkene C-H stretching in the AA+ span 80-mediated formulation. Alkens, benzene and its derivatives stretching occurred near 3,300·㎝−1 in all samples except the non-extrudate ones (UFP-AGR).
The other prominent peaks at 1,700 - 1,500·㎝−1 for all extrudate sample (UFP-AGR-ESF) with chemical formulation possess the characteristics of methylene and methyl bending. The peak region between 3,500 - 3,000·㎝−1 is related to C-H, OH compounds (SP2), which we attribute to the nature of the organic compounds in the formulated sample. Peak regions at < 2,000·㎝−1 represent the carbonyl group compounds and the =C bonds in the aromatic rings and aromatic CH bonds on substituted rings (Silverstein et al., 2005).
The peaks in the region < 1,500·㎝−1 are related to carbon–oxygen bonds (CO) in ethers, esters, and carboxylic acids and are indicative of a wide variety of metabolites, such as tannins, flavonoids, and anthraquinones (Correia et al., 2013).
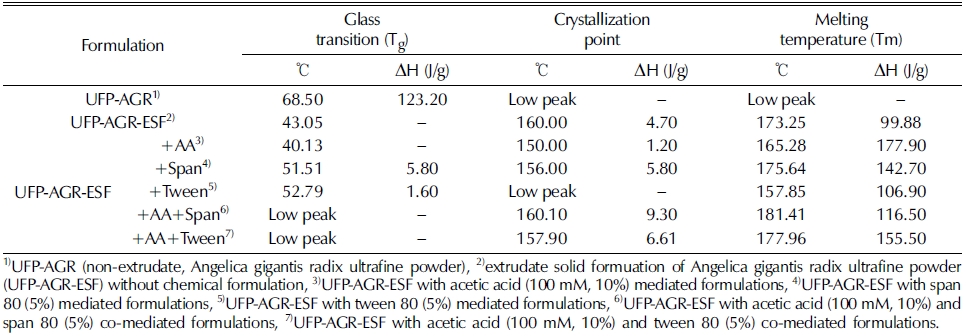
In the DSC analysis, we determined the glass transition temperature (Tg) as well as the crystallization and melting temperature peaks (Table 3).

Glass transition, crystallization point and melting temperature of extrudate solid formuation of Angelica gigantis radix ultrafine powder (UFP-AGR-ESF) by different chemical formulation.
The extrudate solid formuation of Angelica gigantis radix ultrafine powder (UFP-AGR-ESF) had a lower Tg (43.05℃) compared to the non-extrudate of Angelica gigantis radix ultrafine powder, UFP-AGR (68.5℃) and had a with higher ΔH value of 123.2 J/g.
It is generally accepted that amorphous materials have a lower Tg compared to crystalline materials (Yoshioka et al., 1994). The Tg of the extrudate sample appeared as very weak transitions, as more crystalline materials act as physical crosslinks that restrain the mobility of the amorphous regions (Zeleznak and Hoseney, 1987).
Changes in the XRD pattern may occur as a result of core compound-excipient (acid-surfactant emulsifier) interactions. Such alterations include the conversion to a unique polymorphic form, or amorphous to crystalline transitions.
Fig. 2 shows the XRD diffractogram of the extrudate solid formuation of Angelica gigantis radix ultrafine powder (UFP-AGR-ESF) that reveals XRD pattern termed a “halo” which is a gradual rise and fall of the baseline with no distinct peaks (Williams et al., 2012). It provides important insight, based on the degree of long-range order present, into the extent and nature of the crystallinity and microstructure. XRD patterns of crystalline forms show strong diffraction peaks, whereas amorphous states exhibit diffuse and halo diffraction patterns.
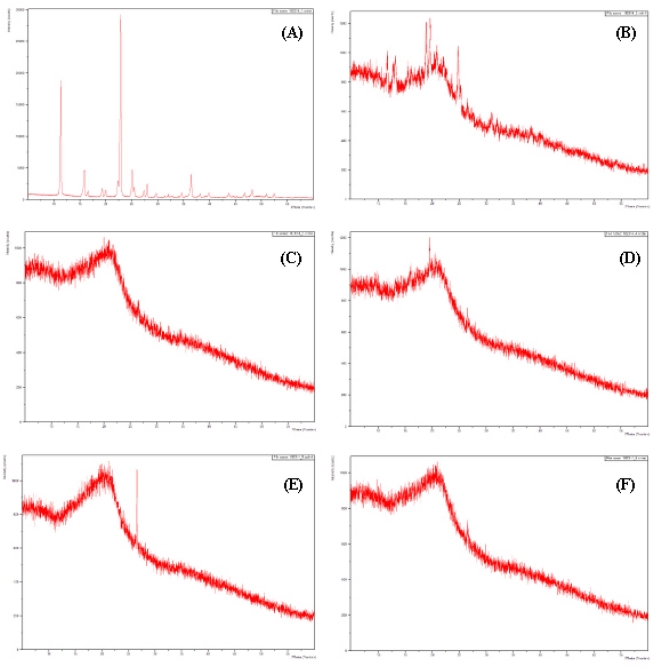
The XRD diffractogram of extrudate solid formuation (UFP-AGR-ESF) by different chemical formulation.(A); Standard courmarin, (B); non-extrudate, Angelica gigantis radix ultrafine powder (UFP-AGR), (C); extrudate solid formuation of Angelica gigantis radix ultrafine powder (UFP-AGR-ESF) without chemical formulation, (D); UFP-AGR-ESF with acetic acid (100 mM, 10%) mediated formulations, (E); UFP-AGR-ESF with acetic acid (100 mM, 10%) and span 80 (5%) co-mediated formulations, (F); UFP-AGR-ESF with acetic acid (100 mM, 10%) and tween 80 (5%) co-mediated formulations.
The presence of a large number of peaks of different intensities in the diffractogram suggests the presence of unidentified complex substances in the extrudate solid formulation.
The acetic acid- and span-mediated solid formulations showed sharp diffraction peaks at angles between 25℃ and 30℃ with a lower degree of diffraction among the formulations.
HME involves the application of pressure and agitation through an extrusion channel to mix materials together, subsequently forcing them out through a die to form an amorphous solid (Wilson et al., 2012).
3. Total phenol content, total flavonoid content, DPPH radical scavenging activity and contents of active compounds in extrudate solid formuation of Angelica gigantis radix ultrafine powder with chemical formulation.
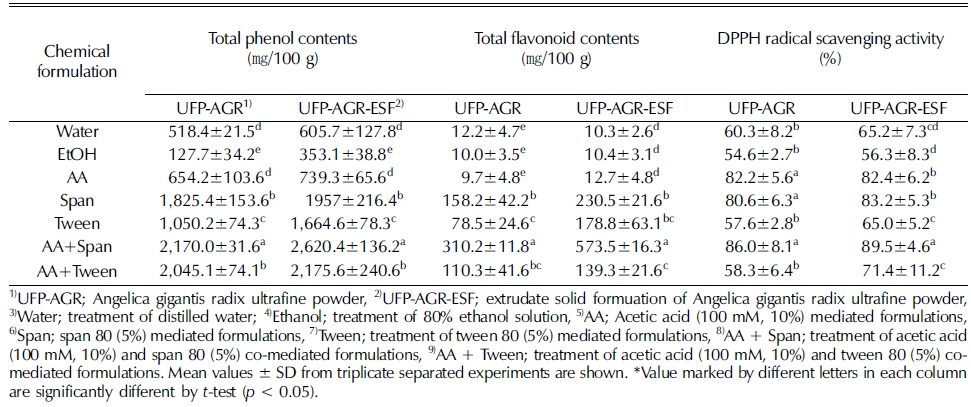
HME showed a positive effect on total phenolic content and antioxidative activity (Ti et al., 2015; Chalermchaiwat et al., 2015), it was reason may be that hydrolysis of polyphenols bound to fiber and/or proteins occurred and the extraction rate of the polyphenols increased (Morales et al., 2015). High extrusion temperatures may lead to degradation of phenolic compounds or cause structural changes (Obradović et al., 2014). Therefore, phenolic compounds may become chemically less reactive or less extractable due to certain degree of polymerization. (Obradović et al., 2014). The optimum combination of process with HME and chemical formulation could be achieved to maximize the total phenolic content and antioxidative activity of chemical formulated extruded products.
In our experiment, higher amounts of total phenolic and total flavonoid content were extracted from the extrudate solid formuation of Angelica gigantis radix ultrafine powder (UFP-AGR-ESF) compared to the non-extrudate of Angelica gigantis radix ultrafine powder (UFP-AGR) (Table 4). Also, DPPH radical scavenging activity of the extrudate solid formuation of Angelica gigantis radix ultrafine powder (UFP-AGR-ESF) was different for each chemical formulation (Fig. 5). DPPH radical scavenging activity was increased in the extrudate solid formulation and then increased again in the chemical (acetic acid and/ or span 80) mediated formulations (Table 4).

Total phenol content, total flavonoid content and DPPH radical scavenging activity of extrudate solid formuation of Angelica gigantis radix ultrafine powder (UFP-AGR-ESF) by different chemical formulation.
The high level of compression and shear forces exerted on the crystalline structure of phenolic molecules lead to their disruption and defibration and the formation of an amorphous structure (Jurisic et al., 2015).
Studies report that HME enhances the dissolution rate of poorly water-soluble compounds (Hülsmann et al., 2000). Piao et al. (2015) showed that HME solid formulation of AGR enhanced the bioavailability in rats. Moreover, Hagi and Hatami (2010) determined higher levels of flavonoid were produced by the use of an acid-mediated solution from vegetables and medicinal plants.
Additionally, Davidov-Pardo et al. (2011) reported that a lower pH is associated with greater stability of flavonoids and their isomers from plant materials.
In our experiment, a lipophilic surfactant showed superior efficiency in the extraction of bioactive compounds over the hydrophilic surfactant in both extrudate solid formuation of Angelica gigantis radix ultrafine powder (UFP-AGRESF) and non-extrudate of Angelica gigantis radix ultrafine powder (UFP-AGR).
In the span 80 surfactant-mediated aqueous solution, the crosslinks formed with polar and non-polar molecules of polyphenolic compounds, and the formation of hydrogen bonds results in the attainment of higher levels of extraction. This enhanced extraction by lipophilic surfactant might be explained on the basis of attractive and repulsive intermolecular interactions. The lipophilic surfactant works by lowering the interfacial tension and reducing the Laplace pressure and stress (Morsy, 2014).
However, studies show that non-ionic hydrophilic surfactants may be more effective than lipophilic surfactants in extracting active compounds, e.g., from apple juice (Hosseinzadeh et al., 2013; Sharma et al., 2015). It is assumed that the types of surfactant that are used will depend upon the nature of the molecules in food compounds, i.e., either polar or non-polar.
HME increases the reactive surface areas of compounds and destructure the fiber matrix, thus will be cause decursin and decursinol angelate to be released into solution. Therefore, the most likely explanation for the enhanced active compound extraction from the extrudate sample is the disruption of cell wall structure (Piao et al., 2015).
It is demonstrated that the application of chemical (surfactant) and physical cross-linking (hot melt extrusion) methods (CPC) by HME was successfully developed to produce an amorphous nano dispersion from solid extrudate of poorly water-soluble AGN active compounds. Physicochemical analysis by FTIR, DSC, and XRD revealed detailed insight into the amorphization of crystalline materials. The finding can be used to establish parameters for the development of a controlled delivery system for the active compounds from AGN.
ACKNOWLEDGEMENTS
This work was supported by 2016 Research Grant (520160205) from the Kangwon National University.
References
-
Agrawal, S, Ashokraj, Y, Bharatam, PV, Pillai, O, Panchagnula, R, (2004), Solid-state characterization of rifampicin samples and its biopharmaceutic relevance, European Journal of Pharmaceutical Science, 22, p127-144, 15158898.
[https://doi.org/10.1016/j.ejps.2004.02.011]

- Ansel, HC, (1985), Introduction to pharmaceutical dosage forms, Lea and Febiger, Philadelphia. PA, USA, p147-149.
-
Bae, IY, Lee, JY, Kwak, BY, Lee, HG, (2011), Estrogenic effects of various extracts from Chamdanggui(Angelica gigas Nakai) and Sogdan(Phlomis umbrosa Turcz), Food Science and Biotechnology, 20, p1113-1118.
[https://doi.org/10.1007/s10068-011-0151-1]

-
Baird, JA, Taylor, LS, (2012), Evaluation of amorphous solid dispersion properties using thermal analysis techniques, Advance Drug Delivery Reviews, 64, p396-421, 21843564.
[https://doi.org/10.1016/j.addr.2011.07.009]

-
Braca, A, Fico, G, Morelli, I, de Simone, F, Tome, F, de Tommasi, N, (2003), Antioxidant and free radical scavenging activity of flavonol glycosides from different Aconitum species, Journal of Ethnopharmacology, 86, p63-67, 12686443.
[https://doi.org/10.1016/S0378-8741(03)00043-6]

-
Chalermchaiwat, P, Jangchud, K, Jangchud, A, Charunuch, C, Prinyawiwatkul, W, (2015), Antioxidant activity, freegamma-aminobutyric acid content, selected physical properties and consumer acceptance of germinated brown rice extrudatesas affected by extrusion process, LWT-Food Science and Technology, 64, p490-496.
[https://doi.org/10.1016/j.lwt.2015.04.066]

-
Choi, KO, Lee, I, Paik, SYR, Kim, DE, Lim, JD, Kang, WS, Ko, S, (2012), Ultrafine Angelica gigas powder normalizes ovarian hormone levels and has antiosteoporosis properties in ovariectomized rats: Particle size effect, Journal of Medicinal Food, 15, p863-872, 23039111.
[https://doi.org/10.1089/jmf.2011.2047]

-
Cocchi, M, Foca, G, Lucisano, M, Marchetti, A, Pegani, MA, Tassi, L, Ulrici, A, (2004), Classification of cereal flours by chemometric analysis of MIR spectra, Journal of Agricultural and Food Chemistry, 52, p1062-1067, 14995098.
[https://doi.org/10.1021/jf034441o]

-
Correia, LP, Procopio, JVV, de Santana, CP, Santos, AFO, Cavalcante, HMM, Macedo, RO, (2013), Characterizationof herbal medicine with different particle sizes using pyrolysis GC/MS, SEM, and thermal techniques, Journal of ThermalAnalysis and Calorimetry, 111, p1691-1698.
[https://doi.org/10.1007/s10973-011-2129-x]

-
Davidov-Pardo, G, Arozarena, I, Marin-Arroyo, MR, (2011), Stability of polyphenolic extracts from grape seeds after thermaltreatments, European Food Research and Technology, 232, p211-220.
[https://doi.org/10.1007/s00217-010-1377-5]

- Fox, SC, (2014), Remington education pharmaceutics, Published by Pharmaceutical Press, London, England, p28-53.
-
Ghimeray, AK, Sharma, P, Phoutaxay, P, Salitxay, T, Woo, SH, Park, SU, Park, CH, (2014), Far infrared irradiation alterstotal polyphenol, total flavonoid, antioxidant property and quercetin production in tartary buckwheat sprout powder, Journal of Cereal Science, 59, p167-172.
[https://doi.org/10.1016/j.jcs.2013.12.007]

-
Hagi, G, Hatami, A, (2010), Simultaneous quantification offlavonoids and phenolic acids in plant materials by a newlydeveloped isocratic high-perfomance liquid chromatography approach, Journal of Agricultural and Food Chemistry, 58, p10812-10816, 20919719.
[https://doi.org/10.1021/jf102175x]

- Harper, JM, (1992), A Comparative analysis of single and twinscrew extruders, Kokini, JL, et al. (ed.), Food extrusionscience and technology, Marcel Dekker, New York. NY, USA, p139-149.
-
Hasenhuettl, GL, Harel, RW, (2008), Food emulsifiers and their applications, 2nd ed., Springer Science, New York. NY, USA, p11-37.
[https://doi.org/10.1007/978-0-387-75284-6_2]

- Holmberg, K, Jonsson, B, Kronberg, B, Lindman, B, (2003), Surfactants and polymers in aqueous solution, John Wiley and Sons, Chichester, England, p16-99.
-
Hosseinzadeh, R, Khorsandi, Hemmaty, S, (2013), Study of the effect of surfactants on extraction and determination of polyphenolic compounds and antioxidant capacity of fruits extracts, PLoS one, 8, pE57353, http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0057353 (cited by 2018 Feb 7).
[https://doi.org/10.1371/journal.pone.0057353]

-
Hu, J, Johnston, KP, Williams, RO, (2004), Nanoparticle engineering processes for enhancing the dissolution rates of poorly water soluble drugs, Drug Development and Industrial Pharmacy, 30, p233-245, 15109023.
[https://doi.org/10.1081/DDC-120030422]

-
Hulsmann, S, Backensfeld, T, Keitel, S, Bodmeier, R, (2000), Melt extrusion-an alternative method for enhancing thedissolution rate of 17β-estradiol hemihydrate, European Journalof Pharmaceutics and Biopharmaceutics, 49, p237-242, 10799815.
[https://doi.org/10.1016/S0939-6411(00)00077-1]

- Jurišić, V, Julson, JL, Krićka, T, Ćuric, D., Voća, N, Karunanithy, C., (2015), Effect of extrusion pretreatment on enzymatic hydrolysis of Miscanthus for the purpose of ethanol production, Journal of Agricultural Science, 7, p132-142.
-
Kang, TC, Hwang, IK, Park, SK, An, SJ, Yoon, DK, Moon, SM, Lee, YB, Sohn, HS, Cho, SS, Won, MH, (2001), Chronological changes of N-methyl-D-aspartate receptors and excitatory amino acid carrier 1 immunoreactivities in CA1 area and subiculum after transient forebrain ischemia, Journal of Neurocytology, 30, p945-955, 12626876.
[https://doi.org/10.1023/A:1021832004954]

-
Khoddami, A, Wilkes, MA, Robert, TH, (2013), Techniques for analysis of plant phenolic compounds, Molecules, 18, p2328-2375, 23429347.
[https://doi.org/10.3390/molecules18022328]

-
Kim, KM, Kim, MJ, Kang, JS, (2009), Absorption, distribution, metabolism, and excretion of decursin and decursinol angelate from Angelica gigas Nakai, Journal of Microbiology and Biotechnology, 19, p1569-1572, 20075620.
[https://doi.org/10.4014/jmb.0905.05028]

-
Kralova, I, Sjőblom, J, (2009), Surfactants used in food industry: A review, Journal of Dispersion Science and Technology, 30, p1363-1383.
[https://doi.org/10.1080/01932690902735561]

-
Lawrence, MJ, Rees, GD, (2012), Microemulsion-based media as novel drug delivery system, Advance Drug Delivery Reviews, 64, p175-193.
[https://doi.org/10.1016/j.addr.2012.09.018]

-
Lee, SH, Shin, DS, Kim, JS, Oh, KB, Kang, SS, (2003), Antibacterial coumarins from Angelica gigas roots., Archives ofPharmacal Research, 26, p449-452.
[https://doi.org/10.1007/bf02976860]

-
Lee, SY, Lee, JJ, Nam, SY, Kang, WS, Yoon, IS, Cho, HJ, (2017b), Fabrication of polymer matrix-free nanocomposites based on Angelica gigas Nakai extract and their application to breast cancer therapy, Colloids and Surfaces B: Biointerfaces, 159, p781-790, 28886514.
[https://doi.org/10.1016/j.colsurfb.2017.08.040]

-
Lee, SY, Nam, SY, Choi, YH, Kim, MJ, Koo, JS, Chae, BJ, Kang, WS, Cho, HJ, (2017a), Fabrication and characterizations ofhot-melt extruded nanocomposites based on zinc sulfate monohydrate and soluplus., Applied Sciences, 7, p902, http://www.mdpi.com/2076-3417/7/9/902/htm (cited by 2018 Jan 6).
[https://doi.org/10.3390/app7090902]

-
Ma, Y, Jung, JY, Jung, YJ, Choi, JH, Jeong, WS, Song, YS, Kang, JS, Bi, K, Kim, MJ, (2009), Anti-inflammatory activities of coumarins isolated from Angelica gigas Nakai on LPSstimulated RAW 264.7 cells, Journal of Food Science and Nutrition, 14, p179-187.
[https://doi.org/10.3746/jfn.2009.14.3.179]

-
Maniruzzaman, M., Rana, MM., Boateng, JS, Mitchell, JC, Douroumis, D., (2013), Dissolution enhancement of poorly water-soluble APIs processed by hot-melt extrusion using hydrophilic polymers, Drug Development and Industrial Pharmacy, 39, p218-227, 22452601.
[https://doi.org/10.3109/03639045.2012.670642]

-
McClements, DJ, Xiao, H, (2012), Potential biological fate ofingested nanoemulsions: Influence of particle characteristics, Food and function, 3, p202-220, 22105669.
[https://doi.org/10.1039/C1FO10193E]

-
Merisko-Liversidge, EM, Liversidge, GG, (2008), Drug nanoparticles: Formulating poorly water-soluble compounds, Toxicologic Pathology, 36, p43-48, 18337220.
[https://doi.org/10.1177/0192623307310946]

-
Morales, JO, McConville, JT, (2011), Manufacture and characterization of mucoadhesive buccal films, European Journal of Pharmaceutics and Biopharmaceutics, 77, p187-199, 21130875.
[https://doi.org/10.1016/j.ejpb.2010.11.023]

-
Morales, P, Cebadera-Miranda, L, Caamara, RM, Reis, FS, Barros, L, Berrios, JDJ, Ferreira, ICFR, Camara, M, (2015), Lentil flour formulations to develop new snack-typeproducts by extrusion processing: Phytochemicals and antioxidantcapacity, Journal of Functional Foods, 19, p537-544.
[https://doi.org/10.1016/j.jff.2015.09.044]

- Morsy, SMI, (2014), Role of surfactants in nanotechnology and their applications, International Journal of Current Microbiology and Applied Science, 3, p237-260.
-
Murdande, SB, Pikal, MJ, Shanker, RM, Bogner, RH, (2010), olubility advantage of amorphous pharmaceuticals: I.A thermodyanamic analysis, Journal of Pharmaceutical Science, 99, p1254-1264, 19697391.
[https://doi.org/10.1002/jps.21903]

-
Nam, SY, Lee, SY, Kim, JJ, Kang, WS, Yoon, IS, (2018), Polydopamine-coated nanocomposites of Angelica gigas Nakai extract and their therapeutic potential for triple-negative breast cancer cells, Colloids and Surfaces B: Biointerfaces, 165, p74-82, 29454167.
[https://doi.org/10.1016/j.colsurfb.2018.02.014]

- Obradović, V, Babić, J, Šubarić, D, Ačkar, D, Jozinović, A, (2014), Improvement of nutritional and functional properties of extruded food products, Journal of Food and Nutrition Research, 53, p189-206.
-
Piao, J, Lee, JY, Weon, JB, Ma, CJ, Ko, HJ, Kim, DD, Kang, WS, Cho, HJ, (2015), Angelica gigas Nakai and soluplusbased solid formulations prepared by hot-melting extrusion: Oral absorption enhancing and memory ameliorating effects, PLoS ONE, 10, pe0124447, http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0124447 (cited by Jan 25).
[https://doi.org/10.1371/journal.pone.0124447]

-
Repka, MA, Battu, SK, Upadhye, SB, Thumma, S, Crowley, MM, Zhang, F, Martin, C, McGinity, JW, (2007), Pharmaceutical applications of hot-melt extrusion: Part II, Drug Development and Industrial Pharmacy, 33, p1043-1057, 17963112.
[https://doi.org/10.1080/03639040701525627]

-
Rosen, MJ, (2004), Surfactants and interfacial phenomena, 3rd ed., John Wiley and Sons, Hoboken. NJ, USA, p52-95.
[https://doi.org/10.1002/0471670561]

- Sebestyen, E., (1974), Grain storage: Problems of grains preservation in storage facilities, Journal of Flour Animal Feed Milling, 10, p24-25.
-
Sharma, S, Kori, S, Parmar, A, (2015), Surfactant mediatedextraction of total phenolic contents(TPC) and antioxidantsfrom fruits juices, Food Chemistry, 185, p284-288, 25952870.
[https://doi.org/10.1016/j.foodchem.2015.03.106]

- Silverstein, RM, Webster, FX, Kiemle, DJ, (2005), Spectrometric identification of organic compounds, 7th ed., John Wiley and Sons, Hoboken. NM, USA, p425-456.
- Singleton, VL, Rossi, JA, (1965), Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents, American Journal of Enology and Viticulture, 16, p144-458.
-
Ti, H, Zhang, R, Zhang, M, Wei, Z, Chi, J, Deng, Y, Zhang, Y, (2015), Effect of extrusion on phytochemical profiles in milled fractions of black rice, Food Chemistry, 178, p186-194, 25704700.
[https://doi.org/10.1016/j.foodchem.2015.01.087]

-
Tita, B, Fulias, A, Bandur, G, Marian, E, Tita, D, (2011), Compatibility study between ketoprofen and pharmaceutical excipients used in solid dosage forms, Journal of Pharmaceuticaland Biomedical Analysis, 56, p221-227, 21665404.
[https://doi.org/10.1016/j.jpba.2011.05.017]

-
Vichapong, J, Sookserm, M, Srijesdaruk, V, Swatsitang, P, (2010), High performance liquid chromatographic analysis ofphenolic compounds and their antioxidant activities in rice varieties, LWT-Food Science and Technology, 43, p1325-1330.
[https://doi.org/10.1016/j.lwt.2010.05.007]

-
Williams, RO III, Watts, AB, Miller, DA, (2012), Formulatingpoorly water soluble drugs, Springer, New York. NY, USA, p45-300.
[https://doi.org/10.1007/978-1-4614-1144-4]

-
Wilson, M, Williams, MA, Jones, DS, Andrews, GP, (2012), Hot-melt extrusion technology and pharmaceutical application, Therapeutic Delivery, 3, p787-797, 22838073.
[https://doi.org/10.4155/tde.12.26]

-
Yan, JJ, Kim, DH, Moon, YS, Jung, JS, Ahn, EM, Baek, NI, Song, DK, (2004), Protection against β-amyloid peptide-induced memory impairment with long-term administration of extract ofAngelica gigas or decursinol in mice, Progress in Neuro-Psychopharmacology and Biological Psychiatry, 28, p25-30, 14687853.
[https://doi.org/10.1016/S0278-5846(03)00168-4]

-
Yoshioka, M, Hancock, BC, Zografi, G, (1994), Crystallizationof indomethacin from the amorphous state below and above its glass transition temperature, Journal of Pharmacology, 83, p1700-1705, 7891297.
[https://doi.org/10.1002/jps.2600831211]

- Zeleznak, KJ, Hoseney, RC, (1987), The glass transition in starch, Cereal Chemistry, 64, p121-124.