
Effects of Catechin-rich Green Tea Extract on the MMP-1 Activity of HaCaT Keratinocyte Cells and on UVB-induced Skin Damage in Hairless Mice
 ; Yang Chun Park*, **
; Yang Chun Park*, ** ; Bok Kyu Kim**
; Bok Kyu Kim** ; Jeong June Choi*** ; Geon Seek Ryu****
; Jeong June Choi*** ; Geon Seek Ryu**** ; Seung Hyung Kim**
; Seung Hyung Kim**
This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Skin is an organ that protects the human body from various environmental stimuli that can induce immune system activation. Skin aging can be largely divided into two categories: physiological aging, which is caused by the a decreased physiological function of the skin and structural changes with aging, and photoaging, which is caused by the chemical stress induced by external stimuli such as ultraviolet (UV) radiation.
The objective of this study was to investigate the anti-wrinkle and UV protective effect of catechin-rich green tea extract (CGTE) in activated keratinocyte (HaCaT cells) and UV-induced skin damage in hairless mice. The results showed that CGTE inhibits the tumor necrosis factor-alpha interferon-gamma (TNF-α + IFN-γ)-induced expression of matrix metalloproteinase (MMP)-1 in HaCaT cells. In addition, the CGTE treatment significantly reduced wrinkle formation, epidermal thickness, collagen deposition, and transepidermal water loss in dorsal skin irradiated with UVB. However, the β-glucosidase activity was significantly increased. The CGTE treatment inhibits mRNA expression and enzyme activity of MMP-2 and MMP-9 in the dorsal skin irradiated with UVB.
It is expected that CGTE can be effectively used as a functional food and cosmetic ingredient to improve skin moisture retention and reduce wrinkle formation.
Keywords:
Catechin-rich Green Tea Extract, UV-induced Skin Damage, Metalloproteinases, Anti-wrinkleINTRODUCTION
Given the increasing interest in cosmetic functional foods that affect skin aesthetics, multiple studies, including cell-level studies, animal studies, and clinical trials, have been performed to identify the ingredients of cosmetic functional foods (Lee, 2008).
The skin is an organ that protects the human body from various environmental stimuli which can induce activation of the immune system. Skin aging can be largely divided into two categories: physiological aging, which is caused by the decreased physiological function of the skin and structural changes with aging, and photoaging, which is caused by chemical stress created from external stimuli such as ultraviolet (UV) radiation.
Exposure of the skin to UV rays increases the risk of skin damage and promotes the aging process (i.e. erythema, edema, pigmentation, roughness, loss of elasticity, and wrinkles) (Chung, 2003). UV light in the atmosphere can be further divided into UVA (315 - 400㎚) and UVB (280 - 315㎚) based on their wavelengths. UVA penetrates more deeply than UVB and therefore is absorbed less in the epidermis as compared to the dermis; UVB induces various changes to the epidermis which can result in the expression of matrix metalloproteinases (MMPs) to promote photoaging, as well as other diseases such as skin cancer (Soter, 1990; Oh et al., 2006).
(-)-Epigallocatechin-3-gallate (EGCG) is a type of catechin-the key ingredient of green tea-and is the most common polyphenol with 200 - 300 ㎎ in a cup of green tea. It has a strong antioxidative effect: 25 fold greater than vitamin E-a well-known antioxidant-and 100 fold greater than vitamin C (Kang et al., 1999; Khan et al., 2006). Previous studies have assessed the role of EGCG in anti-inflammation (Youn, 2007), tumorigenesis and tumor suppression both in vivo and in vitro (Chen et al., 1998), antioxidation (Ha and Kim, 2005), anti-obesity, anti-mutation, and antibacterial effects (Cabrera et al., 2006). In addition, previous animal model studies demonstrate that local treatment or oral administration of polyphenols from green tea or EGCG have both an immunosuppressive and protective effect against tumorigenesis driven by UVB (Rutter et al., 2003; Yusuf et al., 2007).
In this study, in order to investigate the effect of CGTE on improving skin damage, we confirmed a suppression of MMP-1 expression in a keratinocyte (HaCaT) cell line, assessed the effect of CGTE injection on wrinkle formation and moisture retention using a hairless mouse-based photoaging model, and performed histological analysis of mouse back tissue.
MATERIALS AND METHOD
1. Preparation of CGTE
Dried green tea (Camellia sinensis) leaves were purchased from Boseong, Korea in 2016. Green tea leaves (1㎏) were extracted with water at 60℃ for 6 h, twice. The water extract was concentrated under reduced pressure, which was suspended in hot water, and then subjected to Diaion HP-20 column chromatography (Mitsubishi, Tokyo, Japan), eluted with aqueous ethanol (0, 10, 20, 30, 100%), stepwisely. Further purification of the combined 20% and 30% fractions together on Diaion HP-20 column chromatography repeatedly yielded highly pure CGTE (52 g) as a white powder (Choi and Ryu, 2014).
2. HPLC Analysis of CGTE
Contents of individual catechins were determined by HPLC analysis on an Agilent 1220 Infinity LC system (Agilent Technologies Inc., Santa Clara, CA, USA), equipped with a ultra-violet (UV) detector. The analytical experiment of whole extract and CGTE was performed by comparison to tea catechin standards in running condition as shown in Table 1.
An HPLC analysis of CGTE revealed that it consisted of EGC (epigallocatechin, 0.1%), EC (epicatechin, 0.2%), EGCG (97.2%), and ECG (epicatechin gallate, 2.5%), and caffeine-free.
3. Cell culture
Human keratinocytes (HaCaT) were obtained from the Korean cell line bank (Seoul National University, Seoul, Korea). Dulbecco's modified Eagle’s medium (DMEM, Gibco BRL, Gaithersburg, MD, USA) supplemented with 10% inactivated fetal bovine serum (FBS, Gibco BRL, Gaithersburg, MD, USA) containing 500㎍/㎖ penicillin and 500㎍/㎖ streptomycin (GIBCO), and cells were incubated at 37℃ in a humidified atmosphere with 5% CO2. Media were changed every 3 days.
4. MTT assay
Cell viability assay was measured using a quantitative colorimetric assay with thiazolyl blue tetrazolium bromide (MTT, Amresco, OH, USA), showing the mitochondrial activity of living cells. HaCaT cells (1 × 106 cells/㎖) were seed in 96-well plates. After drug treatment as indicated, cells were incubated with MTT (final concentration 0.5㎎/㎖) for 4 h at 37℃. The reaction was terminated by addition of 100㎕ DMSO. Cell viability was measured by an ELISA reader (Molecular Device LLC., Sunnyvale, CA, USA) at 540㎚ excitatory emission wavelength.
5. Western blot analysis
The HaCaT cells were cultured in 60㎜ diameter culture dishes (5 × 106 cells/㎖) and pretreated with TNF-α + IFN-γ (10 ng/㎖) complex various concentrations of CGTE (50, 100 and 200㎍/㎖). After 6 h incubation, cell extracts were prepared using RIPA buffer (Cell Signaling, Danvers, MA, USA) that was supplemented with 1 protease inhibitor cocktail and 1 mM phenyl methyl sulfonyl fluoride.
Protein concentrations of cell lysates were determined with Bradford protein assay (Bio-Rad, Hercules, CA, USA) and 20㎍ of proteins were resoved by 10% SDS/PAGE. The gels were transferred to Polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA) and reacted with appropriate primary antibodies. The membrane was blocked with 5% nonfat milk in Trisbuffered saline with Tween 20 buffer (150 mM NaCl, 20 mM Tris-HCl, and 0.05% Tween 20, pH 7.4). After blocking, the membrane was incubated with primary antibodies for 24 h. Immunostaining with secondary antibodies was detected using Chemi-Doc (Bio-Rad, Hercules, CA, USA) enhanced chemiluminescence substrate and detected with Alpha Imager HP.
6. MMP-1 production
HaCaT cells were cultured in 24 well plates (1 × 106 cells/well), TNF-α + IFN-γ pretreated with CGTE (10, 50, 100, 200㎍/㎖) for 24 h, and exposed to UVB. The production of MMP-1 was determined using a Fluorokine E Human Active MMP-1 Fluorescent Assay Kit (R&D Systems, Minneapolis, MN, USA).
7. UVB irradiation and induced skin wrinkle
Male mice were divided into five groups. all animal procedures were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee (DJUARB2017-033) of the Korea Research Institute of Bioscience and Biotechnology (Daejeon, Korea). The first group did not receive any exposure or treatment and served as control (Nr). The remaining animals were divided into the UVB exposure alone (CT), and the UV + retinoic acid (0.01%), the UV + CGTE 100㎎/㎏, and the UV+CGTE 50㎎/㎏.
The mice in CT, retinoic acid, CGTE 100 and 50 ㎎/㎏ were exposed to a UVB (15 W type, UV maximum wave length 312 ㎚ UV intensity 100㎼·㎝-2, Ieda Boeki Co., Tokyo, Japan) every alternate day. HR-1 mice were irradiated with 100 mJ·㎝-2 UVB radiation (1 minimal erythematal dose = 100 mJ·㎝-2 daily for the first week and from week 2 to week 9, UVB radiation was irradiated three times a week at 200 mJ·㎝-2.
8. Wrinkle Measurement
Skin condition was assessed by photographing the mouse dorsal skin at the end of the experiment to measure the formation of wrinkles. Skin replicas were prepared using a DETAX System II (MIXPAC) and Double-Stick Disc (3M, Carl-Schurz-Straße 1, Neuss, Germany) after UVB exposure.
After the experiment, the double skin disc was attached to the mouse skin, and the mixed solution was sprayed using DETAX System II. After 2-3 minutes, the completely hardened disc was removed and the degree of wrinkling was observed.
9. Measurments water content of stratum corneum
After leaving the area for 30 minutes or more in a room where constant temperature and humidity (temperature 22 ± 1℃, humidity 60 ± 5%) is maintained. After Corneometer from Courage-Khazaka (Koln, Germany), CM825 probe was gently pressed against the skin surface of the back area and recorded as the skin moisture content.
10. Activity of ceramide metabolism enzyme
The separated skin was pulverized with PBS supplemented with 0.1 m PMSF and centrifuged at 10,000 × g for 5 minutes at 4℃. To separated supernatant 50㎕ was added by reaction at 37℃ for 60 minutes citrate-phosphate buffer (pH 5.6, 5 mM sodium taurocholate) containing 0.5 mM 4-methyllumbelliferyl-β-D-glucopyranoside (4-MUG). The reaction is terminated by adding 1,250㎕ of 200 mM carbonate-bicarbonate buffer (pH 10.5). After, the fluorescence intensity of 4-MU (4-methyllumbelliferone) converted from 4-MUG was measured by spectrofluorimeter (excitation = 360㎚, emission = 450㎚) (Hitachi 300, Tokyp, Japan).
11. Quantitative real-time PCR assay
Total RNA was extracted from cells using TRIzol System (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The expression of MMP-1, MMP-2, MMP-9 mRNA was determined by real-time-PCR using Applied Biosystems 7500 Real-Time PCR system (Applied Biosystems, Forster, CA, USA).
The mRNA expression level of proinflammatory cytokines-related genes were quantified via real-time PCR using a SYBR (Applied Biosystems, Grand Island, NY, USA) Green Master Mix. The real-time PCR cycling conditions were as follows: 10 min at 94℃, followed by a total of 45 cycles (15 s at 94℃, 60 s at 60℃). The quantity of each transcript was normalized to the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The primers used in the experiments are listed in Table 2.
12. Histopathology
The skin tissues obtained from the mice were fixed, embedded in paraffin blocks and cut into 5-㎛ thick sections. The skin sections were deparaffinized and stained with hematoxylin and eosin (H&E) (Sigma-Aldrich Co., St. Louis, MO, USA) and Masson’s Trichrome staining.
13. Statistical analysis
All data are shown as the means ± standard error (S.E.). The results of the experimental and control groups were compared for statistical significance (p < 0.05) using paired T-test statistical method by SPSS (IBM SPSS Statistics Version 19.0 Statistic Software, SPSS, Inc., Chicago, IL, USA) for Win 12.0 for summary data.
RESULTS AND DISCUSSIONS
1. Cytotoxicity and suppressive effect of MMP-1 expression
An assessment of cytotoxicity in the HaCaT cell line-to evaluate the effect of CGTE on improving skin wrinkles– showed ≥ 80% cell viability under a maximum concentration of ≥ 400㎍/㎖) CGTE, suggesting no cytotoxicity (Fig. 2A).
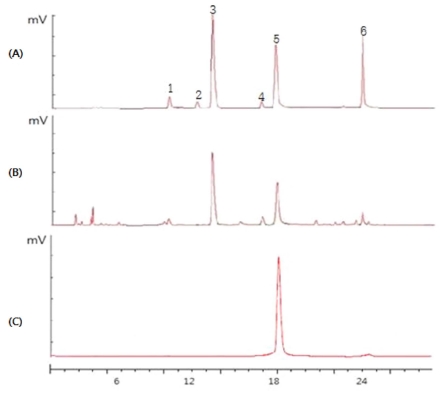
HPLC chromatogram of A) standard 5 catechins and caffeine in green tea and of B) whole extract of green tea and C) CGTE. Peaks: 1; EGC, 2;catechin, 3; caffeine, 4; EC, 5; EGCG, and 6; ECG.
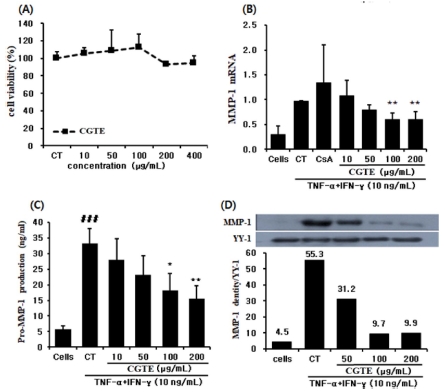
Effect of CGTE on cell viability and MMP-1 expression by TNF-α + IFN-γ treatment in HaCaT cells. HaCaT cells were incubated with TNF-α + IFN-γ (10 ng/㎖) in CGTE (10, 50, 100, 200 and 400 ㎍/㎖) for 24 h. (A) Cell viability was estimated by MTT assay, (B) The expression of MMP-1 mRNA levels in each sample was analyzed by real-time PCR, (C) The supernatants were collected and subjected to enzyme-linked immunosorbent assay (ELISA), (D) The cell lysates were analyzed by Western blotting with anti-MMP-1 anti-YY-1. Anti-YY-1 was used as a control. Values are expressed as means ± S.E. from two independent experiments (*p < 0.05, **p < 0.01). ### p < 0.001 compared with normal group.
Wrinkles are formed by MMPs that are activated via different stimuli [i.e. UV light, oxidative stress, and tumor necrosis factor-α (TNF-α)] (Steenport et al., 2009). MMP-1 is a protein-degrading enzyme that specifically affects collagen; suppressing the activation of MMP-1 is known to improve elasticity to the skin and prevent wrinkle formation (Kondo, 2000).
CGTE-based TNF-α + interferon-gamma (IFN-γ) (10㎍/㎖) treatment in HaCaT cells resulted in significant suppression (≥40%) of MMP-1 mRNA expression in both 200 and 100㎍/㎖ groups as compared to the control group (Fig. 2B). Furthermore, expression of the MMP-1 protein increased by ≥ 5-fold in the control group (33.1 ± 4.87 ng/㎖) as compared to the normal group (5.71 ± 1.22 ng/㎖), but was significantly decreased (by 52.8% and 45.0%) in CGTE 200 and 100㎍/㎖ concentrations, respectively (Fig. 2C).
Moreover, Western blot results demonstrated that, in terms of MMP-1 expression, the band density (BD) value in the control group was 55.3, indicating a ≥ 12-fold increase compared to 4.5 of the normal group. Meanwhile, MMP-1 expression of the experimental group was suppressed in CGTE 200, 100, and 50㎍/㎖ concentrations by 82.0%, 82.4%, and 43.5%, compared to the control group (Fig. 2D). These findings suggest that suppressing the expression of MMP-1, yields positive effects on improving skin wrinkles.
2. Wrinkle measurement in HR-1 mice, and changes in epithelial tissue thickness and collagen fibers
After emitting UVB onto HR-1 mice, the depth of back wrinkles was measured at different time points (4, 6, 8, and 10 weeks after UVB emission) using a DermoBella 3D skin analyzer (DermoBella, Cabo de Santo Agostinho, Brazil). UVB emission resulted in remarkably increased wrinkle formation and depth in the control group, compared to the normal group. Meanwhile, the groups with CGTE 100 and 50㎎/㎏ injections exhibited significantly reduced wrinkle formation and depth compared to the control group (Fig. 3A).
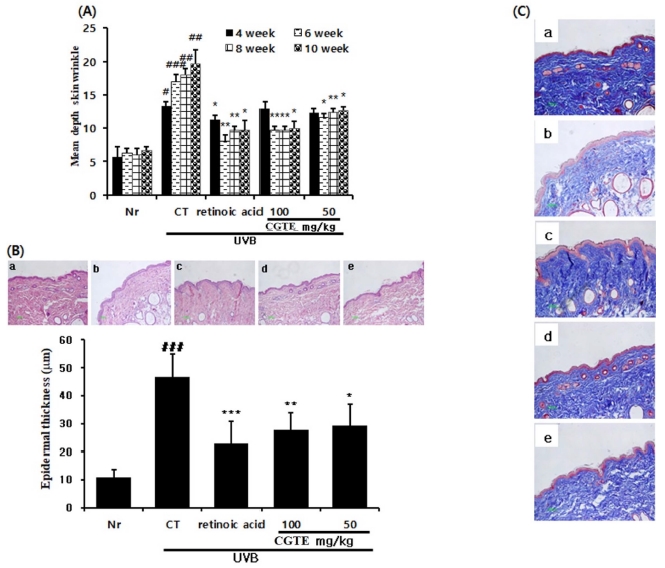
Effect of CGTE on UVB-induced skin wrinkles, epidermal thickening, and histological changes. (A) The skin wrinkle depths of hairless mice were measured at 4, 6, 8, and 10 weeks of UVB radiation, (B) Hematoxylin and eosin (H&E) staining and (C)Masson’s Trichrome (M-T) staining of UVB-irradiated hairless mice skin. Original magnification 100x. a; HR-1 normal, b; UVB-control, c; UVB-retinoic acid 0.01%, d; UVB-CGTE 100㎎/㎏, e; UVB-CGTE 50㎎/㎏. Values are means ± S.E. for 3 mice. Significantly different from UVB/vehicle control treatment (*p < 0.05, **p < 0.01, ***p < 0.001). # p < 0.05, ## p < 0.01, ### p < 0.001 compared with normal group.
The back tissues of HR-1 mice were stained using the hematoxylin and eosin (H&E) staining protocol, and the thickness of epithelial tissue was measured. Compared to the normal group, the control group exhibited 4-fold thicker epithelial tissue. On the contrary, the groups with CGTE 100 and 50㎎/㎏ injections exhibited significantly reduced epithelial tissue thickness compared to the control group (Fig. 3B).
Additionally, the changes in collagen fiber were assessed via Masson’s trichrome staining. The staining intensity in the control group was decreased, suggesting that collagen fiber was largely degraded, and wrinkle formation was accelerated. On the other hand, the groups with CGTE 100 and 50㎎/㎏ injections exhibited an increased amount of collagen fiber compared to the control group, suggesting the recovery was caused by CGTE (Fig. 3C).
3. Effects on retaining skin moisture
The loss of water content in the epidermis is an index used to evaluate the integrity of the skin barrier, including its overall function, recovery after damage, and its protective role against stimuli such as UV light. The Transepidermal Water Loss (TEWL) score, therefore, can be used to evaluate the moisture-retaining function of the skin and the presence of damage to the skin barrier (Berardesca and Maibach, 1990; Levin and Maibach, 2005).
Human skin is constantly exposed to various external factors including UV exposure, which promotes wrinkle formation and pigmentation (Imokawa et al., 1995). In addition, it induces skin dryness from the loss of water content due to the destruction of the skin layer structure caused by reduced levels of ceramide. In order to prevent the loss of ceramide, use of sunscreen (UV blocker) and consumption of functional foods or medication are becoming more common.
Furthermore, society has demonstrated a growing interest in dietary habits (i.e. consumption of specific nutrients or foods) to promote ceramide production and prevent skin damage (Boelsma et al., 2001).
In order to assess the effect of CGTE and its skin moisturizing effect against skin dryness induced by UVB emission, TEWL and the activity of β-glucosidase-which is an enzyme related to ceramide-has been evaluated. TEWL in the control group significantly increased compared to the normal group, while the groups with CGTE 100 and 50㎎/㎏ injections exhibited significantly reduced TEWL compared to the control group (Fig. 4A).
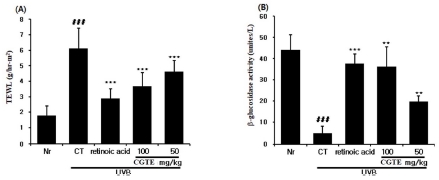
Effect of CGTE on the UVB-induced skin barrier function perturbation. (A) TEWL and (B) β-glucosidase was measured as a marker of the skin barrier function.Values are expressed as means ± S.E. from two independent experiments (**p < 0.01, ***p < 0.001). ### p < 0.001 compared with normal group.
Assessment of β-glucosidase activity showed that the control group exhibited significantly reduced activity of β-glucosidase compared to the normal group, while the activity significantly increased in the groups with CGTE 100 and 50㎎/㎏ injections compared to the control group (Fig. 4B). These outcomes indicate that consumption of CGTE (and consequent prevention of TEWL and suppression of ceramide reduction) can contribute to the improvement of damage to the skin barrier function induced by UVB.
4. Suppressive effects on skin MMP-2, MMP-9 expression
Deficiency of collagen, which is the key ingredient of the dermis, due to aging is a root cause of wrinkle formation, and photoaged skin exhibits wrinkle formation due to increased expression of MMPs (i.e. MMP-1, -2, and -9) which break down collagen. Measurement of MMP-2 and MMP-9 mRNA from isolated back tissue of mice showed that the expression of MMP-2 mRNA significantly increased in the control group compared to the normal group, but significantly decreased in the group with CGTE 100㎎/㎏ injection compared to the control group (Fig. 5A).
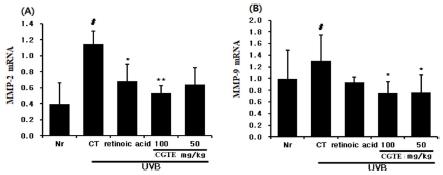
Effect of CGTE on MMP-2 and MMP-9 mRNA expression in animal skin exposed to UVB. After 10 weeks of UVB radiation the expression of MMP-2 (A) and MMP-9 (B) mRNA levels in each sample was analyzed by real-time PCR. Values are expressed as means ± S.E. from two independent experiments (*p < 0.05, **p < 0.01). # p < 0.05 compared with normal group.
Similarly, the expression of MMP-9 mRNA significantly increased in the control group compared to the normal group, but significantly decreased in the groups with CGTE 100 and 50 ㎎/㎏ injection compared to the control group (Fig. 5B).
Acknowledgments
This work was carried out with fund by the 2016 Export Strategy and Technology Development Project (PJ116133-3), supported by the Ministry of Agriculture, Food and Rural Affairs, Republic of Korea.
REFERENCE
- Berardesca, E, and Maibach, HI., (1990), Transepidermal water loss and skin surface hydration in the non invasive assessment of stratum corneum function. Dermatosen in Beruf und Umwelt, Occupation and Environment, 38, p50-53.
-
Boelsma, E, Hendriks, HF, and Roza, L., (2001), Nutritional skin care: Health effects of micronutrients and fatty acids, American Journal of Clinical Nutrition, 73, p853-864.
[https://doi.org/10.1093/ajcn/73.5.853]

-
Cabrera, C, Artacho, R, and Gimenez, R., (2006), Beneficial effects of green tea-a review, Journal of the American College of Nutrition, 25, p79-99.
[https://doi.org/10.1080/07315724.2006.10719518]

-
Chen, ZP, Schell, JB, Ho, CT, and Chen, KY., (1998), Green tea epigallocatechin gallate shows a pronounced growth inhibitory effect on cancerous cells but not on their normal counterparts, Cancer Letters, 129, p173-179.
[https://doi.org/10.1016/s0304-3835(98)00108-6]

- Choi, SW, and Ryu, SY., (2014), Mass production of highly pure epigallocatechin gallate, Korea, Patents. 1016594230000.
-
Chung, JH., (2003), Photoaging in Asians, Photodermatology, Photoimmunology and Photomedicine, 19, p109-121.
[https://doi.org/10.1034/j.1600-0781.2003.00027.x]

- Ha, JS, and Kim, JH., (2005), Protective effect of EGCG against reactive oxygen species-induced stress, International Journal of Oral Biology, 30, p77-84.
-
Imokawa, G, Takema, Y, Yorimoto, Y, Tsukahara, K, Kawai, M, and Imayama, S., (1995), Degree of ultraviolet-induced tortuosity of elastic fibers in rat skin is age dependent, Journal of Investigative Dermatology, 105, p254-258.
[https://doi.org/10.1111/1523-1747.ep12317607]

- Kang, JH, Pack, YK, Chung, ST, and Row, KH., (1999), Extraction and purification of EGCG from green tea, Korean Journal of Biotechnology and Bioengineering, 14, p517-522.
-
Khan, N, Afaq, F, Saleem, M, Ahmad, N, and Mukhtar, H., (2006), Targeting multiple signaling pathways by green tea polyphenol (-)-epigallocatechin-3-gallate, Cancer Research, 66, p2500-2505.
[https://doi.org/10.1158/0008-5472.can-05-3636]

-
Kondo, S., (2000), The roles of cytokines in photoaging, Journal of Dermatological Science, 23, p30-36.
[https://doi.org/10.1016/s0923-1811(99)00076-6]

- Lee, SJ., (2008), Novel natural products as active material for beauty food, Food Science and Industry, 40, p10-18.
-
Levin, J, and Maibach, H., (2005), The correlation between transepidermal water loss and percutaneous absorption: An overview, Journal of Controlled Release, 103, p291-299.
[https://doi.org/10.1016/j.jconrel.2004.11.035]

-
Oh, JH, Kim, A, Park, JM, Kim, SH, and Chung, AS., (2006), Ultraviolet B-induced matrix metalloproteinase-1 and –3 secretion are mediated via PTEN/Akt pathway in human dermal fibroblasts, Journal of Cellular Physiology, 209, p775-785.
[https://doi.org/10.1002/jcp.20754]

-
Rutter, K, Sell, D, Fraser, N, Obrenovich, M, Zito, M, Starke-Reed, P, and Monnier, VM., (2003), Green tea extract suppresses the age-related increase in collagen cross-linking and fluorescent products in C57BL/6 mice, International Journal for Vitamin and Nutrition Research, 73, p453-460.
[https://doi.org/10.1024/0300-9831.73.6.453]

- Soter, NA., (1990), Acute effects of ultraviolet radiation on the skin, Seminars in Dermatology, 9, p11-15.
-
Steenport, M, Khan, KM, Du, B, Barnhard, SE, Dannenberg, AJ, and Falcone, DJ., (2009), Matrix metalloproteinase(MMP)-1 and MMP-3 induce macrophage MMP-9: Evidence for the role of TNF-a and cyclooxygenase-2, Journal of Immunology, 183, p8119-8127.
[https://doi.org/10.4049/jimmunol.0901925]

- Youn, HS., (2007), Anti-inflammatory effects of resveratrol, (-)-epigallocatechin-3-gallate and curcumin by the modulation of toll like receptor signaling pathways, Korean Journal of Food Science and Technology, 39, p481-487.
-
Yusuf, N, Irby, C, Katiyar, SK, and Elmets, CA., (2007), Photoprotective effects of green tea polyphenols, Photodermatology, Photoimmunology and Photomedicine, 23, p48-56.
[https://doi.org/10.1111/j.1600-0781.2007.00262.x]

