
Changes in the Antioxidant Potential of Persimmon Peel Extracts Prepared by Different Extraction Methods
 ; Sung Yong Hwa*** ; Hee Ok Bang* ; Dong Moon Han* ; Ji Yeong Jeon* ; Se Kyung Hwa*, †
; Sung Yong Hwa*** ; Hee Ok Bang* ; Dong Moon Han* ; Ji Yeong Jeon* ; Se Kyung Hwa*, †
This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Astringent persimmon (Diospyros kaki Thunb. cv. Kojongsi) peels are by-products of dried persimmons. This study aimed to evaluate the antioxidant activities of Kojongsi persimmon peel (KPP) extracts prepared by 15 different extraction methods: 5 heating durations (0.5 - 2.5 h) at 3 heating temperatures (50, 70, and 90℃).
An increase in heating temperature increased the antioxidant effect of KPP extracts. Those prepared by heating at 1 h had the highest total phenol content, regardless of the heating temperature. In addition, the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity and cell-protective effects against H2O2-induced oxidative stress were dependent on the total phenol contents of the extract. However, the KPP-induced increased in catalase expression was dependent on heating temperature and duration.
These results suggest that extraction by heating at 90℃ for 1 h may enhance KPP’s antioxidant effects, which mainly involve non-enzymatic antioxidant systems.
Keywords:
Kojongsi, Persimmon, Antioxidant, Oxidative StressINTRODUCTION
Diospyros kaki, a plant species in the family Ebenaceae, is cultivated in East Asian countries such as Korea, Japan, and China for its edible fruit, the persimmon. Persimmon is used as a traditional medicine for conditions such as coughing, hypertension, and frostbites (Yaqub et al., 2016). Various phenolics, including gallic acid, tannin, catechin, epicatechin, quercetin, chlorogenic acid, and caffeic acid, have been identified in persimmons (Butt et al., 2015; Yaqub et al., 2016). Persimmons are generally classified into two groups: sweet (non-astringent) and astringent persimmons, depending on the degree of astringency. Most native cultivars in Korea, such as Kojongsi, Chengdo-Bansi, Dungsi, Sagoksi, Danseongsi, Weolhasi and so on, are astringent persimmons (Jeon et al., 2014).
The cultivation status of Kojongsi persimmon is 11.5% among all persimmon varieties, but it is known as a special local crop, because most Kojongsi persimmon trees grow in the Gyeongsangnam-do area in Korea (Jeon et al., 2014). Because of its astringent taste, Kojongsi persimmon is processed by drying only the pulp without the peel (gotgam) (Chung et al., 2002). During gotgam production, large quantities of peels from persimmons subjected to deastringency treatment are discarded as a by-product. Previous study has reported that industrial application still is limited due to the lack of physiological study for its by-products, which is mostly discarded without use and cause acetic acid fermentation in 2 - 3 days, causing pollution and odor by acidifying soil and water (Kim and Kim, 2005). Therefore, studies on the physiological activities of persimmon by-products are expected to increase the industrial utilization and the discarded persimmon by-products, thereby contributing to the income increase of cultivated farmers and processing companies.
According to encyclopedic sources Donguibogam and Bencao Gangmu, which comprises several types of medicine predominately practiced in East Asia, persimmon peels have shown efficacy in circulatory diseases such as hypertension and arteriosclerosis, and a chronic disease such as diabetes (Kawase et al., 2003; Kim et al., 2006; Celik and Ercisli, 2008). Phenolic contents vary among different cultivars of fruits and vegetables, and within different tissues. Previous studies have reported that the peels of apples and mangoes contain about two to four times the polyphenolic content of their pulp (Eberhardt et al., 2000; Escarpa and Gonzalez, 2001). Also, the peel of persimmon is known to have a higher total phenolic content and antioxidant capacity than the pulp (Kim et al., 2010; Park et al., 2014; Lee et al., 2015). Peels from dried persimmons may be new functional materials, but only a few studies have investigated whether these peels have biological activity (Seo et al., 2000; Kawakami et al., 2010; Jeong et al., 2018).
Previous studies have evaluated the antioxidative, UV-blocking, and anti-inflammatory effects of Kojongsi persimmon (Jeong et al., 2010; Cho et al., 2011a; Cho et al., 2011b; Jeon et al., 2014). However, the antioxidant activity of Kojongsi persimmon peel extracts is still unclear. Our aims in this study were to compare the antioxidant capacities of Kojongsi persimmon peel extracts prepared by different extraction methods and to investigate their ability to protect human endothelial EA.hy926 cells against oxidative stress.
MATERIAL AND METHODS
1. Reagents.
Folin-Ciocalteu’s phenol reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), pyrogallol, resveratrol, gallic acid, dimethyl sulfoxide (DMSO), and hydrogen peroxide were purchased from Sigma-Aldrich (St. Louis, MO, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from USB (Cleveland, OH, USA). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and trypsin were purchased from Hyclone (Logan, UT, USA). Antibodies against superoxide dismutase 2 (SOD2), catalase (CAT), and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Horseradish peroxidase-conjugated anti-mouse and anti-rabbit IgG antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). All other chemicals were of the highest grade available commercially.
2. Preparation of Kojongsi persimmon peel extracts.
Kojongsi persimmon fruits were produced in Hadong, Gyeongsangnam-do, Korea. In order to ensure the safety of the fruits from pesticides and microorganisms, fruits that had been certified in accordance with Good Agricultural Practices (GAP) were selected. The collected Kojongsi persimmon peels were tested for residual pesticides for use as raw materials for food and cosmetics.
To make gotgam, Kojongsi persimmon is processed from mid-November to the end of November; the Kojongsi persimmon peels were collected on the day of processing. After one day of storage, the peels were washed twice with flowing water and dried at 60℃ for 36 h. Kojongsi persimmon peel extracts (KPP) were extracted at a ratio of dried peel weight of 10 g in 200 ㎖ of primary distilled water. Fifteen samples were prepared with five different heating times (0.5 h, 1 h, 1.5 h, 2 h, and 2.5 h) and three heating temperatures (50 ℃, 70℃, and 90℃). The extracts were filtered under reduced pressure and freeze-dried (for 3 days) to prepare KPP powder.
3. Total phenolic content.
Total phenolic contents were assayed using the Folin-Ciocalteu reagent (Dewanto et al., 2002). First, 125 ㎕ of the sample extract was dissolved in 500 ㎕ of distilled water and 125 ㎕ of Folin-Ciocalteu reagent. The mixture was shaken, followed by the addition of 1.25 ㎖ of 7% Na2CO3; distilled water was then added to adjust the final volume to 3 ㎖, followed by thorough mixing. After incubation in the dark for 90 min, the absorbance of the samples at 760 ㎚ was measured and compared to that of the prepared blank.
Total phenolic contents were expressed as ㎎ of gallic acid equivalents per gram of dry weight (㎎·GAE/g·DW), through a calibration curve prepared using the gallic acid standard.
4. DPPH radical-scavenging activity.
The DPPH radical-scavenging activity was estimated according to the method described by Bios (1958). A 1 ㎖ aliquot of each extract was mixed with 2 × 10-4 M solution of DPPH in methanol. The mixture was shaken vigorously and allowed to rest at room temperature for 30 min in the dark. The absorbance of the resulting solution was then measured at 517 ㎚. The ability to scavenge the DPPH radical was calculated using the following equation:
DPPH scavenging effect (%) = [(A0 − A1)/A0] × 100
In this equation, A0 and A1 are the absorbances of the control and extract samples, respectively. All samples were analyzed in triplicate.
5. Cell culture.
EA.hy926 cells were obtained from the American Type Culture Collection (Bethesda, MD, USA) and cultured in DMEM supplemented with 10% FBS, 100 U/㎖ penicillin, and 100 ㎍/㎖ streptomycin (HyClone, Logan, UT, USA) at 37℃ in a humidified incubator containing 5% CO2. In all in vitro experiments, EA.hy926 cells were used at passages 3-10.
6. Cell viability assay.
Conventional MTT reduction assay was used to determine the viability of EA.hy926 cells. Cells were seeded in 48-well plates and incubated at 37℃ for 24 h. Cells in each well were treated with various concentrations of KPP for 24 h, and then with 200 μM H2O2 for 24 h. Then, MTT solution was added, followed by incubation for 30 min; the resultant formazan crystals were solubilized by adding DMSO. The absorbance of the cell samples at 550 ㎚ was measured using a BioTek Synergy HT microplate reader (Bio-Tek, Winooski, VT, USA).
7. Western blot analysis.
After treatment, EA.hy926 cells were lysed in lysis buffer [120 mM NaCl, 40 mM Tris (pH 8), and 0.1% NP40 (Nonidet P-40)] on ice for 30 min and centrifuged at 12,000 rpm for 20 min. Supernatants were collected and the protein concentrations were measured using a protein assay kit (Pro-Measure, Intron biotechnology, Seongnam, Korea).
Aliquots of the lysates (50 ㎍ of protein from each lysate) were boiled for 5 min and then electrophoresed on 10% SDS-PAGE gels. Then, the proteins were transferred onto PVDF membranes, which were incubated with the primary antibodies. The membranes were then incubated with secondary anti-mouse or anti-rabbit antibodies. Finally, the protein bands were detected using an enhanced chemiluminescence Western blotting detection kit (Biofact, Daejeon, Korea). The integrated optical density for the protein bands was calculated using the Image-J software (U.S. National Institutes of Health, Bethesda, MD, USA), and then, the values were normalized to β-actin levels.
8. Real-time polymerase chain reaction (PCR).
Total RNA was extracted from KPP-treated cells using an RNAiso reagent (Takara Shuzo, Kyoto, Japan) according to the manufacturer’s protocol. The accumulated PCR products were detected directly by monitoring the increase in the reporter dye (SYBR®) signal. The quantity of each transcript was calculated according to the manufacturer’s instructions and normalized to the amount of β-actin, a housekeeping gene.
The primer sets used in the PCR amplification were as follows; CAT (forward; 5'-CTG ACT ACG GGA GCC ACA TC-3', reverse; 5'-CAT CCA GTG ATG AGC GGG TT-3'); SOD2 (forward; 5'-CCA AGA CCT GTA TCT CCA GTC A-3', reverse; 5'-TGA GTG GTA TGT GAC AAC AGC A-3'); β-actin (forward; 5'-GTC ATT CCA AAT ATG AGA TGC GT-3', reverse; 5'-GCT ATC ACC TCC CCT GTG TG-3').
9. Statistical analysis.
The results are expressed as the means ± standard deviation (S.D.). Differences between groups were analyzed via one-way analysis of variance, followed by Dunnett’s Multiple Comparison Test (GraphPad InStat, GraphPad Software, San Diego, CA, USA). p < 0.05 was considered a statistically significant difference.
RESULTS AND DISCUSSION
1. Total phenolic content.
The total phenolic contents of KPP samples prepared using 15 different extracting conditions: five different heating times (0.5 h, 1 h, 1.5 h, 2 h, and 2.5 h) and three heating temperatures [50 (KPPL), 70 (KPPM) and, 90℃ (KPPH)] are shown in Table 1.

Total phenol contents of Kojongsi persimmon peel extracts prepared by treatment at different extracting conditions.
The total phenolic contents of the KPP samples with regards to the heating temperature were ordered as follows: KPPH > KPPM > KPPL; the total phenolic contents of the KPP samples with regards to the heating time were ordered as follows: 1 h > 2.5 h > 2 h > 1.5 h > 0.5 h. Higher temperature conditions showed more total phenol contents and the highest total phenolic content was found in KPP samples prepared by heating treatment at 90℃ and 1 h (KPPH-1h) (1,000.2 ± 72.4 ㎎·GAE/100g). This is similar to the result of the study by Jeong et al. (2018), who demonstrated that the total phenolic content of water-soluble extracts of Cheongdo-Bansi peels was between 1,060.3 ± 126.3 ㎎·GAE/100g. Interestingly, in case of all three heating temperatures, an extracting time of 1 h showed relatively higher total phenolic contents, compared to other extracting conditions.
This observation can be supported by Silva et al. (2007), who indicated that a longer extraction time was not useful to extract more phenolic antioxidants. Also, Salar et al. (2016) has mentioned that extended extraction process might lead to oxidation of phenolic compounds owing to prolonged light or oxygen exposure. Therefore, the increase of heating temperature for KPP may be involved in the upregulation of total phenolic contents but not time-dependently.
2. Antioxidant activity.
The antioxidant capacities of ten KPP extracts (10 ㎎/㎖) measured using the DPPH radical-scavenging activity and SOD-like activity assays are shown in Fig. 1. In the DPPH radical-scavenging activity assay, KPP samples treated at a higher temperature showed stronger radical scavenging activity, and treatment for an extracting time of 1 h showed a higher scavenging activity in case of both KPPH-1h (86.2 ± 1.5%), KPPM-1h (83.0 ± 1.3%), and KPPL-1h (80.3 ± 0.6%) compared to other extracting conditions; this is similar to the results of the total phenolic content. The DPPH radical-scavenging activities of the KPP samples with regards to the heating temperature were ordered as follows; KPPH > KPPM > KPPL, the DPPH radical-scavenging activities of the KPP samples with regards to the heating time were ordered as follows; 1 h > 2.5 h > 2 h > 1.5 h > 0.5 h.
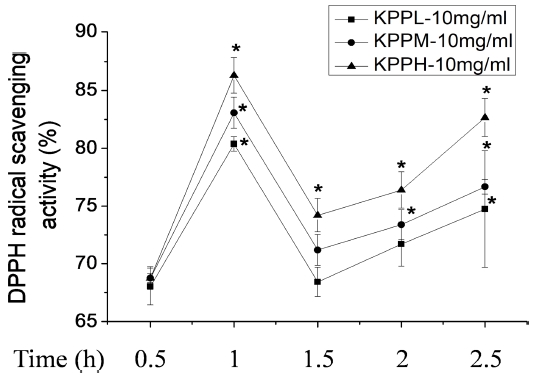
Effects of Kojongsi persimmon peel extracts prepared by treatment at different extracting conditions on DPPH radical-scavenging activity. DPPH radical-scavenging activity. ■; 10 ㎎/㎖ water extract of Kojongsi persimmon peel prepared by heating treatment for 0.5 - 2.5 h at 50℃ (KPPL-10 ㎎/㎖), ●; 10 ㎎/㎖ water extract of Kojongsi persimmon peel prepared by heating treatment for 0.5 - 2.5 h at 70℃ (KPPM-10 ㎎/㎖), ▲; 10 ㎎/㎖ water extract of Kojongsi persimmon peel prepared by heating treatment for 0.5 - 2.5 h at 90℃ (KPPH-10 ㎎/㎖). Means with difference letters of the same column are significantly different at p < 0.05 by Dunnett’s Multiple Comparison Test (DMCT).
This indicates that the DPPH radical-scavenging activities of the ten KPP samples are highly related to their total phenol contents. Bound forms of phenolic have been reported to be converted to free forms by heating, which results in an increased antioxidant capacity (Lee et al., 2006; Lee et al., 2014). Manthey and Perkins-Veazie (2009) have reported that there were the uniform changes in total phenolic content and DPPH antioxidant capacity, relative to cultivar type.
3. Protective effect of KPP against hydrogen peroxide-induced oxidative stress in endothelial cells.
Persimmon peel extract has been reported to protect N18-RE-105 cells from glutamate-induced cytotoxicity, in contrast to persimmon extracts from other parts of the fruit or plant (Lee et al., 2011). Persimmon peel extract has also been reported to protect PC-12 cells from glucose-oxygen-serum deprivation-induced ischemic injury and H2O2-induced oxidative stress (Forouzanfar et al., 2016). These previous studies have indicated that KPP has a potent protective effect against oxidative stress in various cell lines. To determine whether KPP has protective effects against oxidative stress-induced cell death, using the MTT assay, we tested the protective effect of KPP against hydrogen peroxide-induced oxidative stress in endothelial EA.hy926 cells. Because of their location, endothelial cells are easily exposed to oxidative stress; a damaged endothelium is the main cause of several vascular diseases (Haybar et al., 2019).
At first, KPPM-1.5h was chosen as a representative of ten KPP samples, and was tested to determine the concentrations at which the samples were not toxic. KPPM-1.5h had no cytotoxicity against EA.hy926 cells at a concentration of up to 1㎎/㎖, which is the highest concentration of KPP for application to cells (Fig. 2A). Thus, the cells were exposed to 1㎎/㎖ of KPP in subsequent experiments. Additionally, resveratrol (20 μM), a well-known natural antioxidant, was used as the positive control. Oxidative stress (200 μM of H2O2) decreased the viability of EA.hy926 cells to approximately 65% (Fig. 2B). Pretreatment of the cells with the KPP samples increased the viability of EA.hy926 cells exposed to oxidative stress (Fig. 2B). The cell-protective effects (based on the cell viability) of the KPP samples with regards to the heating time were ordered as follows: 1 h > 2.5 h > 2 h > 1.5 h > 0.5 h. Interestingly, the trend of cell viability against H2O2 were also similar to that of total phenol contents and DPPH-radical scavenging activity. This indicate that the polyphenolic contents and the in vitro antioxidant capacity may be the most important factors to decrease oxidative stress-induced cell death.
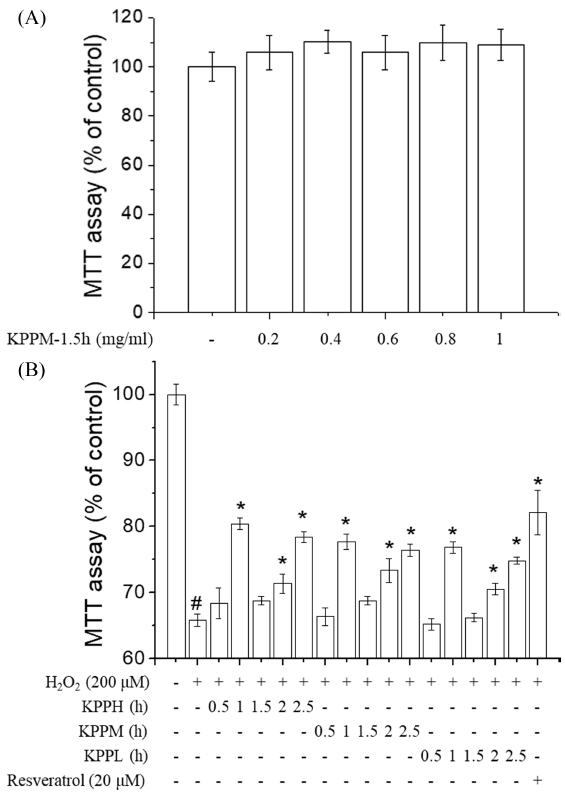
Protective effects of Kojongsi persimmon peel extracts prepared by treatment at different extracting conditions against oxidative stress (H2O2)-induced cellular damage in endothelial EA.hy926 cells. Cells were treated with 0.2–1 ㎎/㎖ of Kojongsi persimmon peel water extract prepared by heating treatment for 1.5 h at 70℃ (KPPM-1.5h). (A); cells were treated with 15 different Kojongsi persimmon peel extracts (1 ㎎/㎖) and resveratrol (20 μM) for 12 h and then incubated with H2O2 for further 24 h, (B); cells viabilities were measured by the MTT assay. Results are presented as the means ± SD of three independent experiments. #p < 0.05; significantly different from the control, *p < 0.05; significantly different from H2O2-treated cells by Dunnett’s Multiple Comparison Test (DMCT).
4. Effect of KPP on antioxidant enzyme expression in endothelial cells.
Mammalian cells are equipped with enzymatic antioxidant defense mechanisms, among which are the superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) enzymes, and non-enzymatic systems such as the vitamins A, C, and E, among others (Hercberg et al., 2004; Halliwell et al., 2005; Yuan et al., 2010; Ighodaro and Akinloye, 2018). The antioxidant enzymes contribute to eliminating radicals such as superoxide () and hydrogen peroxide (H2O2), preventing the formation of highly reactive species and hydroxyl radicals (HO-), which are damaging to cells. To study whether KPP induce the expression of antioxidant enzymes, we examined the effect of KPP on the gene and protein expression of SOD2 and CAT in EA.hy926 cells.
As shown in Fig. 3, treating the cells with KPP samples extracted at higher temperatures and a longer heating time showed a greater effect on the induction of CAT expression, but none of the extracts affected the SOD2 expression in endothelial cells. These results are not consistent with those of the cell viability test against oxidative stress, indicating that the enzymatic antioxidant expression induced by KPP is not the main mechanism for their protective effects against oxidative stress.
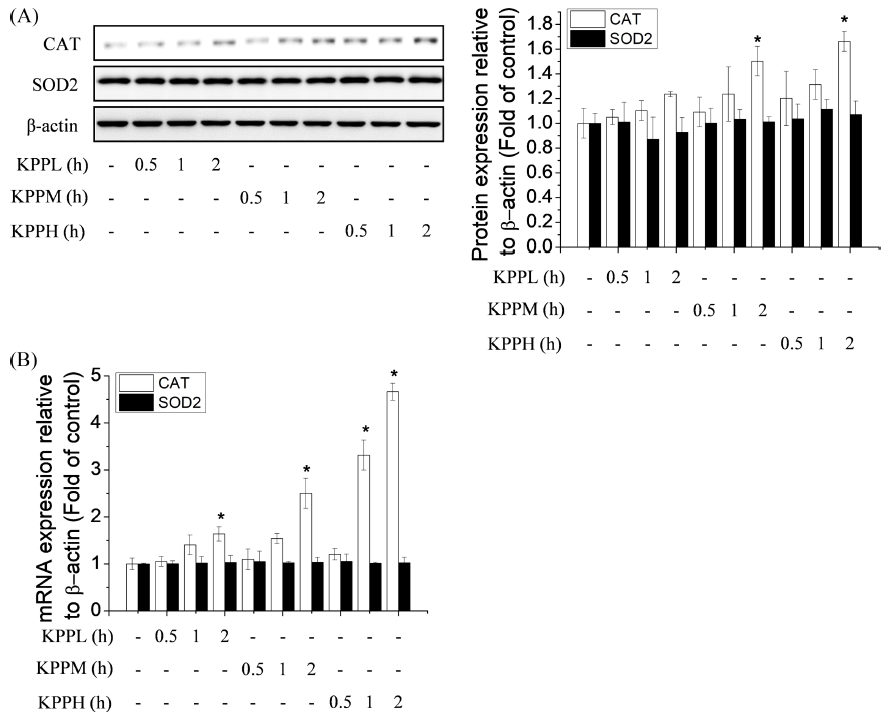
Effects of Kojongsi persimmon peel extracts prepared by treatment at different extracting conditions on antioxidant enzyme expression in endothelial cells. (A); cells were treated with nine different Kojongsi persimmon peel extracts for 24 h, and then, cell lysates were prepared for immunoblotting. The catalase (CAT) and superoxide dismutase 2 (SOD2) protein levels were compared to those of β-actin using the image J software, (B); cells were treated with nine different Kojongsi persimmon peel extracts for 12 h; they were then lysed and total RNA was extracted for CAT and SOD2 analysis. Results are presented as the means ± SD of three independent experiments. *p < 0.05; significantly different from the control by Dunnett’s Multiple Comparison Test (DMCT).
Similarly, Kim et al. (2017) have reported that extracts of persimmon fruits reduced H2O2-induced oxidative stress in Caco-2 human colonic epithelial cells, but these results are not consistent with its effect on the antioxidant activities including CAT and SOD. However, in this study, we only measured mRNA or protein expression levels, not antioxidant activities, which is a study limitation. Therefore, whether KPP affect the related antioxidant activities and the mechanisms involved should be investigated further.
In order to study the antioxidant effects of Kojongsi persimmon peels, a by-product of dried persimmon processing, and optimize the heating temperature and time for extracting water-soluble antioxidants from these peels, fifteen different extracting methods were designed according to five different heating times (0.5 to 2.5 h) at 50, 70, and 90℃. In the overall study of antioxidant activity assays, KPP samples extracted by treatment at 90℃ showed a higher antioxidant effect than those extracted by treatment at lower heating temperatures. The total phenol content and DPPH radical-scavenging activity of KPP showed an increase in case of a heating time of 1 h and 2.5 h, compared to the treatments for other heating times. In the analysis of cell viability under conditions of oxidative stress, KPP significantly increased the viability of H2O2-treated EA.hy926 cells, and the trend of cell viability against H2O2 were also similar to that of total phenol contents and DPPH-radical scavenging activity. However, KPP increased the expression of the antioxidant enzyme SOD2 and CAT in EA.hy926 cells, but the pattern of this increase is not consistent with the cell-protective effects of KPP. This indicates that the antioxidant activity of KPP mainly involves non-enzymatic antioxidant systems. The data from the present study suggest that high-temperature heating is the most effective way to induce the antioxidant effects of KPP, but further study is needed to clear the antioxidant activities of KPP in different heating time.
Acknowledgments
This work carried out with the support of Technology Development Program of Application for Natural Materials by Gyeongnam Technopark, Republic of Korea.
References
-
Blois, MS., (1958), Antioxidant determinations by the use of a stable free radical, Nature, 181, p1199-1200.
[https://doi.org/10.1038/1811199a0]

- Butt, MS, Sultan, MT, Aziz, M, Naz, A, Ahmed, W, Kumar, N, and Imran, M., (2015), Persimmon(Diospyros kaki) fruit: Hidden phytochemicals and health claims, EXCLI Journal, 14, p542-561.
- Celik, A, and Ercisli, S., (2008), Persimmon cv. Hachiya(Diospyros kaki Thunb.) fruit: Some physical, chemical and nutritional properties, International Journal of Food Sciences and Nutrition, 59, p599-606.
- Cho, JK, Jeon, IH, Park, JM, Kim, HS, and Jang, SI., (2011a), Inhibitory effect of persimmon leaf extract on development of atopic dermatitis-like skin lesions, Journal of the Korean Society for Applied Biological Chemistry, 54, p653-657.
- Cho, JK, Park, JM, Jeon, IH, Kim, HS, and Jang, SI., (2011b), Effect of persimmon leaf extract on ultraviolet B-induced inflammation in HaCat keratinocytes and mice, Journal of the Korean Society for Applied Biological Chemistry, 54, p583-590.
- Chung, JY, Kim, KH, Shin, DJ, and Son, GM., (2002), Effects of sweet persimmon powder on the characteristics of bread, Journal of the Korean Society of Food Science and Nutrition, 31, p738-742.
-
Dewanto, V, Xianzhong, W, Adom, KK, and Liu, RH., (2002), Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity, Journal of Agricultural and Food Chemistry, 50, p3010-3014.
[https://doi.org/10.1021/jf0115589]

-
Eberhardt, MV, Lee, CY, and Liu, RH., (2000), Antioxidants activity of fresh apples, Nature, 405, p903-904.
[https://doi.org/10.1038/35016151]

- Escarpa, A, and González, MC., (2001), Approach to the content of total extractable phenolic compounds from different food samples by comparison of chromatographic and spectrophotometer methods, Analytica Chimica Acta, 427, p111-127.
- Forouzanfar, F, Torabi, S, Askari, VR, Asadpour, E, and Sadeghnia, HR., (2016), Protective effect of Diospyros kaki against glucose-oxygen-serum deprivation-induced PC12 cells injury, Advances in Pharmacological Sciences, 2016, 3073078 https://www.hindawi.com/journals/aps/2016/3073078/abs/ (cited by 2019 May 20).
-
Halliwell, B, Rafter, J, and Jenner, A., (2005), Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: Direct or indirect effects? Antioxidant or not?, American Journal of Clinical Nutrition, 81, p268-276.
[https://doi.org/10.1093/ajcn/81.1.268s]

-
Haybar, H, Shahrabi, S, Rezaeeyan, H, Shirzad, R, and Saki, N., (2019), Endothelial cells: From dysfunction mechanism to pharmacological effect in cardiovascular disease, Cardiovascular Toxicology, 19, p13-22.
[https://doi.org/10.1007/s12012-018-9493-8]

- Hercberg, S, Galan, P, Preziosi, P, Bertrais, S, Mennen, L, Malvy, D, Roussel, AM, Favier, A, and Briançon, S., (2004), The SU.VI.MAX study: A randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals, Archives of Internal Medicine, 164, p2335-2342.
- Ighodaro, OM, and Akinloye, OA., (2018), First line defence antioxidants-superoxide dismutase(SOD), catalase(CAT) and glutathione peroxidase(GPX): Their fundamental role in the entire antioxidant defence grid, The American Journal of Medicine, 54, p287-293.
-
Jeon, IH, Kang, HJ, Lee, HS, Shin, JH, Park, YG, Jeong, SI, and Jang, SI., (2014), Antioxidant and anti-inflammatory activities of water-soluble extracts from different parts of Kojongsi persimmon (Diospyros kaki L.), Korean Journal of Food Science and Technology, 46, p505-510.
[https://doi.org/10.9721/kjfst.2014.46.4.505]

- Jeong, DW, Cho, CH, Lee, JS, Lee, SH, Kim, TW, and Kim, DO., (2018), Deastringent peel extracts of persimmon(Diospyros kaki Thunb. cv. Cheongdo-Bansi) protect neuronal PC-12 and SH-SY5Y cells against oxidative stress, Journal of Microbiology and Biotechnology, 28, p1094-1104.
- Jeong, SI, Cho, JK, Mok, JY, Kim, SJ, Park, JM, Jeon, IH, Kim, HS, and Jang, SI., (2010), Antioxidant activity of persimmon leaves during growth, Korean Journal of Pharmacognosy, 41, p255-263.
-
Kawakami, K, Aketa, S, Nakanami, M, Iizuka, S, and Hirayama, M., (2010), Major water-soluble polyphenols, proanthocyanidins, in leaves of persimmon(Diospyros kaki) and their α-amylase inhibitory activity, Bioscience, Biotechnology, and Biochemistry, 74, p1380-1385.
[https://doi.org/10.1271/bbb.100056]

-
Kawase, M, Motohashi, N, Satoh, K, Sahagami, H, Nakashima, H, Tani, S, Shirataki, Y, Kurihara, T, Spengler, G, Wolfard, K, and Molnar, J., (2003), Biological activity of persimmon (Diospyros kaki) peel extracts, Phytotherapy Research, 17, p495-500.
[https://doi.org/10.1002/ptr.1183]

-
Kim, H, Moon, JY, Kim, HJ, Lee, DS, Cho, MJ, Choi, HK, Kim, YS, Mosaddik, A, and Cho, SK., (2010), Antioxidant and antiproliferative activities of mango(Mangifera indica L.) flesh and peel, Food Chemistry, 121, p429-436.
[https://doi.org/10.1016/j.foodchem.2009.12.060]

-
Kim, L, Kim, YY, Kwon, O, and Kim, JY., (2017), Antioxidant activities of ethanolic and acidic ethanolic extracts of astringent persimmon in H2O2-stimulated Caco-2 human colonic epithelial cells, Food Science and Biotechnology, 26, p1085-1091.
[https://doi.org/10.1007/s10068-017-0156-5]

- Kim, SY, Jeong, SM, Kim, SJ, Jeon, KI, Park, EJ, Park, HR, and Lee, SC., (2006), Effect of heat treatment on the antioxidative and antigenotoxic activity of extracts from persimmon(Diospyro kaki L.) peel, Bioscience, Biotechnology, and Biochemistry, 70, p999-1002.
- Kim, YJ, and Kim, BK., (2005), Effect of dietary persimmon peel powder on physico-chemical properties of pork, Korean Journal for Food Science of Animal Resources, 25, p39-44.
-
Lee, I, Lee, BH, Eom, SH, Oh, CS, Kang, H, Cho, YS, and Kim, DO., (2015), Antioxidant capacity and protective effects on neuronal PC-12 cells of domestic bred kiwifruit, Korean Journal of Horticultural Science & Technology, 33, p259-267.
[https://doi.org/10.7235/hort.2015.14123]

-
Lee, MR, Moon, SH, Choi, AR, Lee, SC, Ahn, KH, and Park, HR., (2011), Neuroprotective effects of extracts from Diospyros kaki L. peel, Korean Journal of Food and Cookery Science, 27, p67-73.
[https://doi.org/10.9724/kfcs.2011.27.4.067]

-
Lee, MS, Lee, II, Kim, YG, Kim, YJ, Heo, HJ, and Kim, DO., (2014), Inhibitory effect of the ethyl acetate fraction from astringent persimmon on H2O2-induced oxidative stress in HepG2 cells, Food Science and Biotechnology, 23, p1247-1252.
[https://doi.org/10.1007/s10068-014-0171-8]

-
Lee, SC, Jeong, SM, Kim, SY, Park, HR, Nam, KC, and Ahn, DU., (2006), Effect of far-infrared radiation and heat treatment on the antioxidant activity of water extracts from peanut hulls, Food Chemistry, 94, p489-493.
[https://doi.org/10.1016/j.foodchem.2004.12.001]

- Manthey, JA, and Perkins-Veazie, P., (2009), Influences of harvest date and location on the levels of β-carotene, ascorbic acid, total phenols, the in vitro antioxidant capacity, and phenolic profiles of five commercial varieties of mango(Mangifera indica L.), Journal of Agricultural and Food Chemistry, 57, p10825-10830.
-
Park, JH, Lee, MH, and Park, EJ., (2014), Antioxidant activity of orange flesh and peel extracted with various solvents, Preventive Nutrition and Food Science, 19, p291-298.
[https://doi.org/10.3746/pnf.2014.19.4.291]

-
Salar, RK, Purewal, SS, and Bhatti, MS., (2016), Optimization of extraction conditions and enhancement of phenolic content and antioxidant activity of pearl millet fermented with Aspergillus awamori MTCC-548, Resource-Efficient Technologies, 2, p148-157.
[https://doi.org/10.1016/j.reffit.2016.08.002]

- Seo, JH, Jeong, YJ, and Kim, KS., (2000), Physiological characteristics of tannins isolated from astringent persimmon fruit, Korean Journal of Food Science and Technology, 32, p212-217.
-
Silva, EM, Souza, JNS, Rogez, H, Rees, JF, and Larondelle, Y., (2007), Antioxidant activities and polyphenolic contents of fifteen selected plant species from the Amazonian region, Food Chemistry, 101, p1012-1018.
[https://doi.org/10.1016/j.foodchem.2006.02.055]

-
Yaqub, S, Farooq, U, Shafi, A, Akram, K, Murtaza, MA, Kausar, T, and Siddique, F., (2016), Chemistry and functionality of bioactive compounds present in persimmon, Journal of Chemistry, 2016, 3424025 https://www.hindawi.com/journals/jchem/2016/3424025/abs/ (cited by 2019 May 20).
[https://doi.org/10.1155/2016/3424025]

-
Yuan, G, Sun, B, Yuan, J, and Wang, Q., (2010), Effect of 1-methylcyclopropene on shelf life, visual quality, antioxidant enzymes and health-promoting compounds in broccoli florets, Food Chemistry, 118, p774-781.
[https://doi.org/10.1016/j.foodchem.2009.05.062]
