Anti-hangover and Anti-gout Effects of Asparagus Byproduct Extracts
This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Given the reported pharmacological effects of rutin from asparagus stems and roots, here, we assessed the anti-hangover and anti-gout effects of asparagus stem and root extracts.
We found that rutin amount in ethanol extracts of asparagus stems was 10.15 ㎎/g, and in hydrothermal extracts of asparagus roots it was 3.28 ㎎/g. Alcohol dehydrogenase and aldehyde dehydrogenase activities in asparagus-stem extracts were 80% and 40% of those in the control group, respectively. Following hangover induction in rats using alcohol, an injection of 400 ㎎/㎏ body weight (BW) of the extract significantly reduced the blood alcohol concentration compared to that of the control group. Additionally, blood gamma-glutamyl transpeptidase (GGT), glutamic oxaloacetic transaminase (GOT), and glutamic pyruvic transaminase (GPT) concentrations measured 5 h after alcohol administration revealed that treatment with 400 ㎎/㎏ BW of extract significantly reduced GGT and GOT concentrations compared to the control group, whereas changes in GPT were not significant. Moreover, xanthine oxidase activity after treatment with hydrothermal extracts of asparagus roots was 44.01 ± 0.32%, showing 53.3% inhibition compared to the positive control (allopurinol; 82.50 ± 4.98%). To assess the anti-gout effect of hydrothermal extracts of asparagus roots, we induced gout in rats by intraperitoneal injection of potassium oxonate and measured blood uric acid, creatinine, and blood urea nitrogen concentrations at 3 h post-administration of the extract. The results showed a significant reduction in creatinine and uric acid levels in the treated group compared to the control.
These findings demonstrated the potential of asparagus stem and root extracts as functional ingredients.
Keywords:
Asparagus officinalis L., Anti-hangover Effect, Anti-gout EffectINTRODUCTION
Asparagus (Asparagus officinalis L.) is a perennial plant that belongs to the family Liliaceae, genus Asparagus, and cultivated in tropical, temperate, dry, and desert regions across Europe, North America, South America, Africa, and Oceania (Lee et al., 2015).
In South Korea, cultivation tests were started in 1966, and the cultivation area reached 700 ㏊ in 1968. However, there was little consumer demand owing to poor standards of living and low awareness of Western vegetables. Moreover, because of the long seed-raising period, it requires > 2 years from planting to first harvest, and there are problems with low productivity and underdeveloped cultivation techniques, which lead to significant decreases in the cultivation area.
However, as national income has recently increased, along with interest in well-being and health functionality, the cultivation area has gradually expanded (Ki et al., 2001; Park et al., 2016), with the total cultivation area nationwide currently 85 ㏊, 62% of which is reportedly in Gangwon-do (Kwon et al., 2020).
Compared with most vegetables, asparagus is rich in protein, fat, vitamins, and minerals (Martin-Belloso and Llanos-Barriobero, 2001). Asparagus also contains various physiologically active phytochemicals, including polysaccharides, steroid saponins, flavonoids, dietary fiber, and oligosaccharides, which reportedly exhibit antioxidant, antibacterial, antiepileptic, anticancer, and immunoregulatory effects (Kahlon et al., 2007; Elsaid et al., 2015; Sullivan et al., 2017; Fadda et al., 2018; Wang et al., 2020).
Approximately 23.5% of the whole asparagus is used for food, while 76.5% comprises byproducts, of which 15% are the roots. These asparagus byproducts have traditionally been used to prevent nonspecific inflammatory diseases, kidney or bladder stones, liver disease, bronchial asthma, and gout (Al-Snafi, 2015).
Asparagus stems and roots contain flavonoid-based rutin and polyphenol-based tannins, which exhibit pharmacological effects, including antibacterial and antioxidant, aging prevention, memory enhancement, antiepileptic, antidepressant, anti-inflammatory, and analgesic effects (Jang et al., 2004; Xiong et al., 2011; Fan et al., 2015; Symes et al., 2018; Chitrakar et al., 2019; Zhang et al., 2019). Particularly, rutin is the most abundant flavonoid in asparagus (Tsushida et al., 1994; Wang et al., 2003).
Therefore, in this study, we analyzed the anti-hangover and anti-gout effects of asparagus byproducts in rats to demonstrate the increased utility of extracts of asparagus stems and roots, which are currently considered byproducts.
MATERIALS AND METHODS
1. Preparation of asparagus byproduct (stem and root) extracts
The roots and stems of the Atlas Asparagus (A. officinalis L.) variety were 5 years old and were cultivated in Yanggu, Korea. The roots were collected in September and the stems were collected in October. Stems and roots used in this test were used except for edible sprouts. Asparagus-root hydrothermal extracts were prepared, as follows.
Using optimal extraction conditions and response surface methodology (RSM), 29 g of sample was added to 1ℓ of distilled water, and reflux condensation was performed for 95 min at 100℃ using a Soxhlet extractor (MS-EAM, MISUNG, Yangju, Korea). The extract was centrifuged for 15 min at 3,000 × g, and the supernatant was collected and filtered using Whatman No. 1 filter paper, and freeze-dried (PVTF20R, Ilshinbiobase Co., Ltd., Dongducheon, Korea) to produce the hydrothermal extracts.
The ethanol extracts of asparagus stems were prepared, as follows. Using optimal extraction conditions and RSM, 60 g of sample was added to 1ℓ of 30% fermentation alcohol while shaking at 25℃, and the mixture was incubated to allow extraction for 10 h. The extract was then filtered using Whatman No. 1 filter paper, enriched using a negative-pressure evaporator (Rotavapor R-220SE, BÜCHI Labortechnik AG, Flawil, Switzerland), and dried using a freeze dryer (Ilshinbiobase Co., Ltd., Dongducheon, Korea) to prepare the samples.
2. Analysis of rutin content
Each asparagus extract was dissolved in 70% ethanol to a concentration of 10 ㎎/㎖, sonicated for 10 min, and centrifuged for 20 min at 3,200 × g. After centrifugation, the supernatant was filtered using a 0.45 ㎛ syringe filter, and the filtrate was used as the sample for analysis.
The reference standards for rutin analysis were purchased from Sigma-Aldrich (St. Louis, MO, USA). To construct a calibration curve for quantification, each standard was mixed to obtain a concentration of 100 ㎍/㎖, which was diluted to create concentrations of 100 ㎍/㎖, 250 ㎍/㎖, and 1,000 ㎍/㎖.
3. Anti-hangover effects of asparagus stem extract
ADH activity was measured using an ADH activity assay kit (Sigma-Aldrich Co., St. Louis, MO, USA). ADH produces aldehyde from ethanol, and the kit measures ADH activity based on the production of NADH from NAD.
To measure the ADH activity of the asparagus stem ethanol extract, 50 ㎕ of extract was mixed with 82 ㎕ of ADH assay buffer, 8 ㎕ of developer, and 10 ㎕ of 2 M ethanol, and the mixture was left to react at 37℃ Measurements were taken after 2 min and 7 min, and the amount of NADH produced was measured at a wavelength of 450 ㎚.
ALDH activity was measured using an ALDH activity assay kit (Sigma-Aldrich Co., St. Louis, MO, USA). To measure the ALDH activity of the asparagus stem ethanol extract, 50 ㎕ of extract was mixed with 43 ㎕ of ALDH assay buffer, 2 ㎕ of ALDH substrate mix, and 5 ㎕ of acetaldehyde, and incubated at 37℃. Measurements were taken after 5 min and 8 min, and the amount of NADH produced was measured at a wavelength of 450 ㎚.
For the control group, results were compared with enzyme activity generated after the administration of a commercially available hangover-relief tablet (Coslip Cure Red 487, Herb Cure Co., Ltd., Pocheon, Korea). Activity was evaluated according to the following equation:
Where B represents the amount (nmol) of NADH generated between the initial and final times (T), reaction time represents the difference of the initial and final times (min), and V represents the sample volume (㎖) added to each well.
Sprague-Dawley rats (male; 7 weeks old) were obtained from Koatech Co., Ltd. (Pyeongtaek, Korea) and acclimated for 1 week under constant conditions (temperature, 22 ± 1℃; humidity, 55 ± 3%; light-dark cycle, 12 h) before use in experiments.
Animal experiments were approved by the Chuncheon Bioindustry Foundation Institutional Animal Care and Use Committee (CBF IACUC No. 2020-019).
Rats were weighed before the start of the experiment. After 18 h of fasting, rats were given either the positive control substance (yeast extract, 200 ㎎/㎏ BW) or the asparagus stem ethanol extract (200 and 400 ㎎/㎏ BW) via oral administration.
After 30 min, 3 g/㎏ BW ethanol was administered orally, and blood samples were taken from the tail vein after 0 h, 1 h, 3 h, and 5 h. After collection, the blood was centrifuged at 3,000 × g at 4℃ for 20 min (5810R, Eppendorf, Hamburg, Germany) to isolate serum.
A gas chromatography flame ionization detector (GC-2010, Shimadzu Co., Kyoto, Japan) was used to measure blood alcohol and acetaldehyde concentrations. After mixing 10 ㎕ of isopropanol with 100 ㎕ of serum, 1.2% Triton X-100 and 1.8 g/ℓ acetonitrile in water were added to a total volume of 500 ㎕. The mixture was centrifuged at 4℃ and 60,000 × g for 5 min, and the supernatant was collected for analysis.
At 5 h after ethanol administration, rats were anesthetized and sacrificed, and whole blood was collected from the abdominal aorta and centrifuged (3,000 × g, 20 min, and 4℃) to isolate serum.
A biochemistry analyzer (Konelab 20 B, Thermo Fisher Scientific Inc., Vantaa, Finland) was used to measure the major blood liver enzymes in the serum, gamma(γ)-glutamyl transferase (GGT), glutamic oxaloacetic transaminase (GOT), and glutamic pyruvic transaminase (GPT).
4. Anti-gout effects of asparagus root extract
Xanthine oxidase inhibitory activity was measured using an improved version of the method described previously (Stirpe et al., 1969).
A 1 ㎖ substrate solution containing 2 mM xanthine and 0.1 M potassium phosphate buffer (pH 7.5) was prepared, to which 0.1 ㎖ of xanthine oxidase (Sigma-Aldrich Co., St. Louis, MO, USA) and 0.1 ㎖ of sample at varying concentrations were added. The mixture was incubated for 5 min at 37°C before adding 1 ㎖ of 20% (v/v) trichloroacetic acid to stop the enzyme reaction, after which the mixture was centrifuged (2,800 × g for 15 min, 4℃), the supernatant collected, and the absorbance measured at 292 ㎚.
The positive control was allopurinol (Sigma-Aldrich Co., St. Louis, MO, USA), which is a known xanthine oxidase inhibitor, prepared at a concentration of 200 ㎍/㎖. The rate of enzyme inhibition was expressed as the percentage of uric acid produced in the reaction solution relative to the control:
Sprague-Dawley rats were randomly divided into two groups (n = 7/group), with each group orally administered either the test substance (hydrothermal extract of asparagus root, 100 or 200 ㎎/㎏ BW) or the positive control (allopurinol 50 ㎎/㎏ BW) for 7 days.
The animal experiment was approved by the Chuncheon Bioindustry Foundation Institutional Animal Care and Use Committee (CBF IACUC No. 2020-013).
Rats were weighed before the start of the experiment. After 18 h of fasting to increase the serum urate levels, the rats were administered 200㎎/㎏ BW of potassium oxonate by intraperitoneal injection.
After 1 h, the rats were then orally administered either the hydrothermal extract of asparagus root (100 or 200 ㎎/㎏ BW) or the positive control (allopurinol 50 ㎎/㎏ BW). Following administration of asparagus extract, blood was collected from the tail vein after 1 h and 3 h, and blood samples were centrifuged (5810R, Eppendorf, Hamburg, Germany) at 4℃ and 3,000 × g for 20 min to isolate serum.
To measure the anti-gout effects of the asparagus root extract, a biochemistry analyzer (Konelab 20 B, Thermo Fisher Scientific Inc., Vantaa, Finland) was used to measure serum creatinine, uric acid, and blood urea nitrogen (BUN) levels.
Data were expressed as the means ± standard deviation for each group.
After performing one-way analysis of variance using GraphPad Prism software (v.4.0, GraphPad Software, La Jolla, CA, USA), Tukey’s multiple comparison test was used to determine significant differences between groups, with a p < 0.05 considered significant.
RESULTS
1. Extract yield and rutin content
Using the optimal conditions for RSM, yields of 18.58 ± 0.57% and 22.65 ± 0.57% were achieved for the ethanol extract of asparagus (A. officinalis L.) stem and the hydrothermal extract of asparagus root, respectively.
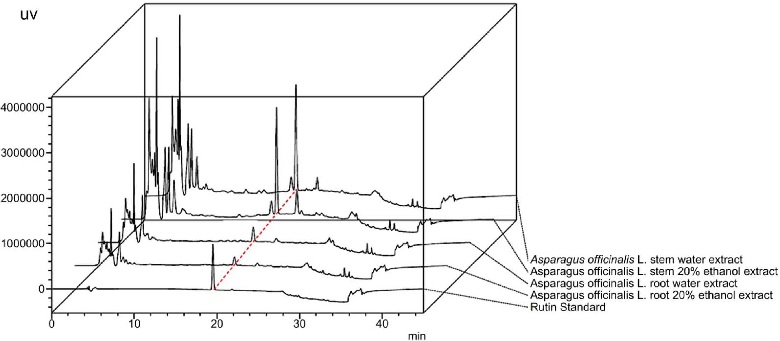
HPLC analysis of extracts showed that rutin eluted within 15 min, and detection patterns between the ethanol extract of asparagus stem and the hydrothermal extract of asparagus root were similar (Fig. 1). Rutin content was 10.15 ㎎/g in asparagus stem extract and 3.28 ㎎/g in the asparagus root hydrothermal extract.
2. Anti-hangover effects of asparagus stem extract
Measurements of changes in blood alcohol concentration indicated a rapid increase up to 1 h after alcohol consumption in the rat hangover model, followed by a decreasing trend (Fig. 2).
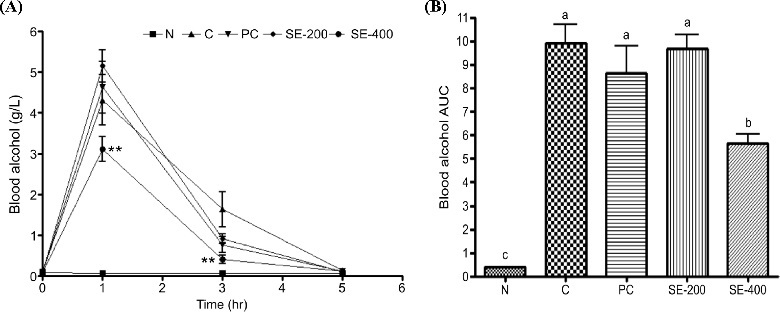
Blood alcohol scavenging activity of stem extract from Asparagus according to changes in (A) blood alcohol concentration and (B) blood alcohol concentration area under the curve (AUC) over time after alcohol administration.Data represent the means ± SD (n = 3). *p < 0.01 vs. control for each time period. Different letters above bar graphs signify differences with a p < 0.05. N; normal, C; control, PC; positive control, SE-200; 200 ㎎/㎏ BW of stem 20% ethanol extract from Asparagus, SE-400; 200 ㎎/㎏ BW of stem 20% ethanol extract from Asparagus.
In the group administered 400 ㎎/㎏ BW asparagus stem extract, a significant decrease in blood alcohol was measured as compared with that of the control group up to 3 h after alcohol consumption. Calculation of the area under the curve (AUC) for blood alcohol concentration over time showed that alcohol consumption increased blood alcohol concentration, followed by a significant decrease of about 45% in the group receiving 400 ㎎/㎏ BW asparagus stem extract. Additionally, blood acetaldehyde concentration increased up to 3 h after alcohol consumption (Fig. 3), whereas groups treated with asparagus stem extract showed a decrease in acetaldehyde concentration 3 h and 5 h after alcohol consumption, although these decreases were not significant.
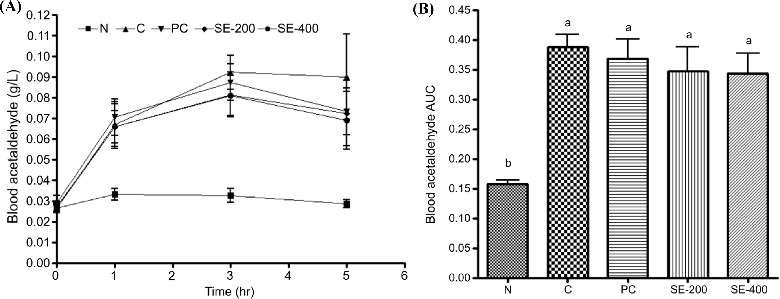
Blood acetaldehyde scavenging activity of stem extract from Asparagus according to the changes in (A) blood acetaldehyde concentration and (B) blood acetaldehyde concentration area under the curve (AUC) over time after alcohol administration.Data represent the means ± SD (n = 3). *p < 0.01 vs. control for each time period. Different letters above bar graphs signify differences with a p < 0.05. N; normal, C; control, PC; positive control, SE-200; 200 ㎎/㎏ BW of stem 20% ethanol extract from Asparagus, SE-400; 200 ㎎/㎏ BW of stem 20% ethanol extract from Asparagus.
Moreover, the AUC showed that blood acetaldehyde concentration decreased over time in the group treated with asparagus stem extract, although the difference was not significant. Measurements of blood GGT, GOT, and GPT concentrations 5 h after alcohol administration revealed a significant increase in GGT and GOT in the control group as compared with the normal group, whereas significant decreases were observed in the positive control group and those treated with 400 ㎎/㎏ BW asparagus extract (Fig. 4). By contrast, GPT concentration was similar in all groups.
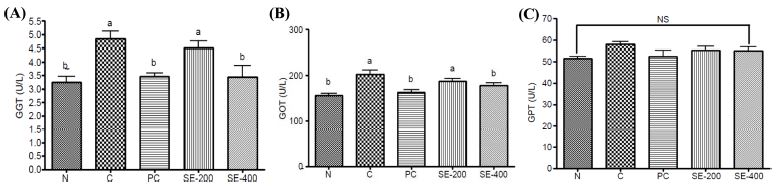
Effect of stem extract from Asparagus on (A) gamma-glutamyl transferase (GGT), (B) glutamic oxaloacetic transaminase (GOT), and (C) glutamate pyruvate transaminase (GPT) concentrations measured after 5 h in alcohol-treated rats.Data represent the means ± SD (n = 3). *Different letters and “NS” (not significant) above the bar graphs indicate significant differences (p < 0.05). N; normal, C; control, PC; positive control, SE-200; 200 ㎎/㎏ BW of stem 20% ethanol extract from Asparagus, SE-400; 200 ㎎/㎏ BW of stem 20% ethanol extract from Asparagus.
3. Anti-gout effect of asparagus root extract
To assess the anti-gout effect of hydrothermal extracts of asparagus roots, we induced gout in rats via intraperitoneal injection of potassium oxonate, followed by administration of the extract and measurement of blood uric acid, creatinine, and BUN concentrations over time.
At 1 h post-potassium oxonate injection, uric acid and BUN levels significantly increased relative to those in the normal group, and the group administered 400 ㎎/㎏ BW of hydrothermal extracts of asparagus roots showed a significant reduction relative to the control group.
Additionally, creatinine concentration, which is an indicator of renal dysfunction, increased slightly in the control group relative to the normal group and tended to decrease in the group receiving asparagus root extract, although there was no significant difference among treatment groups.
At 3 h post-potassium oxonate injection, BUN, creatinine, and uric acid concentrations significantly increased relative to the normal group, whereas the asparagus root extract group showed a significant reduction in creatinine and uric acid concentrations as compared with the control group (Fig. 5).
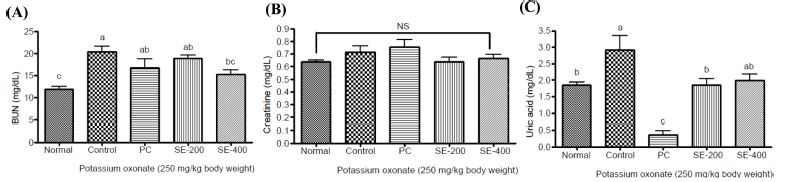
Effect of Asparagus officinalis L. stem extract on urea (A), creatinine (B), and uric acid (C) concentrations after 1 h in potassium oxonate-treated rats.Data represent the means ± SD (n = 3). Different letters above the bar graphs signify differences at p < 0.05. N: normal; C: control; PC: positive control (allopurinol, 50 ㎎/㎏ BW); SE-200: A. officinalis L. stem 20% ethanol extract, 200 ㎎/㎏ BW; SE-400: A. officinalis L. stem 20% ethanol extract, 400 ㎎/㎏ BW.
DISCUSSION
In this study, we assessed the anti-hangover and anti-gout effects of asparagus (A. officinalis L.) stem and root extracts by first analyzing their rutin content and the activities of ADH and ALDH. After inducing hangover or gout in rats, we examined the effect of the rutin-containing stem and root extracts on xanthine oxidase inhibitory activity; changes in blood alcohol, acetaldehyde, uric acid, BUN, and creatinine concentrations; and changes in liver enzymes.
Rutin is the most abundant flavonoid in asparagus (Tsushida et al., 1994; Wang et al., 2003), and Fusi et al. (2003) reported that rutin exhibits a strong antioxidant effect that inhibits oxidation of DNA, proteins, and lipids, and strengthens capillaries. We measured higher rutin content in asparagus stems than roots, consistent with the findings of Kwon et al. (2020) who reported an 8 fold higher rutin content in stems than roots of asparagus. These findings also agree with other reports showing that the rutin content in asparagus differs depending on the variety, cultivation method, and part of the stem (Maeda et al., 2005; Maeda et al., 2010; Motoki et al., 2012a; Motoki et al., 2012b).
Our in vivo results revealed that treatment of alcohol-induced hangover model rats with 400 ㎎/㎏ BW ethanolic asparagus stem extract significantly reduced blood alcohol, GGT, and GOT concentrations, and demonstrated a reduction in acetal- dehyde concentration after alcohol intake relative to the control group.
Most of the alcohol absorbed in the body is absorbed by the stomach and small intestine, after which it is oxidized to acetaldehyde in hepatocytes by ADH in the cytoplasm, cytochrome P4502E1 in the endoplasmic reticulum, and catalase in peroxisomes. Acetaldehyde is subsequently oxidized by ALDH forming acetic acid, which is ultimately excreted in the form of urea and CO2 (Kee et al., 2003; Jung et al., 2004). GGT, GOT, and GPT, which are used as indicators of liver damage, are mostly present in hepatocytes, but released into circulation when hepatocytes are damaged, resulting in increased blood concentrations (Lee et al., 2016).
Lee et al. (2016) reported that aspartate transaminase (AST) concentration increased significantly following alcohol consumption relative to that in a normal group, whereas alanine transaminase (ALT) concentration increased, although not significantly. They also showed that groups administered different concentrations of a black and red ginseng mixture, and a comparison group administered a commercially-available anti-hangover drink, showed no changes in AST and ALT levels previously elevated by alcohol consumption (Lee 2016).
Additionally, Lim et al. (2011) demonstrated that a group treated with a drink containing Bulnesia sarmienti extract showed significant decreases in alkaline phosphatase, GOT, and GPT as compared with a control group administered alcohol.
Furthermore, Kim (2004) reported that GOT and GPT levels elevated after alcohol administration were significantly decreased by kudzu root administration owing to its hepatoprotective effects. When reviewing the above findings and the experimental results of this study, we concluded that the described ethanol extract of asparagus contains functional ingredients that have the potential to provide hangover relief.
Additionally, evaluation of the effects of asparagus root extracts on xanthine oxidase inhibitory activity to evaluate possible anti-gout efficacy, revealed a 53.3% inhibition in activity relative to the positive control group. When uric acid concentration in the blood is abnormally elevated owing to issues with purine metabolism, the low solubility of uric acid causes it to accumulate in joints, resulting in gout (Storch and Feber, 1998).
Xanthine oxidase is involved in purine metabolism, resulting in formation of uric acid from xanthine or hypoxanthine (Kim et al., 1996). Therefore, efforts to identify anti-gout agents usually involve measurements of xanthine oxidase-inhibitory activity (Shin et al., 2013).
Here, we showed that treatment of gout-induced rats with hydrothermal extracts of asparagus roots resulted in significant decreases in blood uric acid and creatinine concentrations 3 h after extract administration.
Nguyen et al. (2004) tested the xanthine oxidase inhibitory activity of root extracts of Asparagus cochinchinensis and reported similar activities at 100 ㎍/mL methanol extract (13.2%), 50% methanol extract (7.4%), and water extract (9.3%), suggesting that the xanthine oxidase inhibitory activity of root extracts of Asparagus spp. is unrelated to solvent polarity. These results demonstrated that asparagus root extracts could improve gout by lowering blood uric acid levels and alleviating diminished renal functions, thereby suggesting the potential of these extracts as a treatment for gout.
Altogether, these findings suggest the potential efficacy of asparagus stem and root extracts as functional ingredients.
References
- Al-Snafi AE. (2015). The pharmacological importance of Asparagus officinalis–A review. Journal of Pharmaceutical Biology. 5:93-98.
-
Chitrakar B, Zhang M and Adhikari B. (2019). Asparagus (Asparagus officinalis): Processing effect on nutritional and phytochemical composition of spear and hard-stem byproducts. Trends in Food Science and Technology. 93:1-11.
[https://doi.org/10.1016/j.tifs.2019.08.020]

-
Elsaid FG, Shati AA and Sarhan MA. (2015). Role of Matricaria recutita L. and Asparagus officinalis L. against the neurotoxicity of diazinon in rats. Journal of Basic and Applied Zoology. 72:26-35.
[https://doi.org/10.1016/j.jobaz.2015.02.002]

-
Fadda A, Barberis A and Sanna D. (2018). Influence of pH, buffers and role of quinolinic acid, a novel iron chelating agent, in the determination of hydroxyl radical scavenging activity of plant extracts by electron paramagnetic resonance(EPR). Food Chemistry. 240:174-182.
[https://doi.org/10.1016/j.foodchem.2017.07.076]

-
Fan R, Yuan F, Wang N, Gao Y and Huang Y. (2015). Extraction and analysis of antioxidant compounds from the residues of Asparagus officinalis L. Journal of Food Science and Technology. 52:2690-2700.
[https://doi.org/10.1007/s13197-014-1360-4]

-
Fusi F, Saponara S, Pessina F, Gorelli B and Sgaragli G. (2003). Effects of quercetin and rutin on vascular preparations: A comparison between mechanical and electrophysiological phenomena. European Journal of Nutrition. 42:10-17.
[https://doi.org/10.1007/s00394-003-0395-5]

-
Jang DS, Cuendet M, Fong HHS, Pezzuto JM and Kinghorn AD. (2004). Constituents of Asparagus officinalis evaluated for inhibitory activity against cyclooxygenase-2. Journal of Agricultural and Food Chemistry. 52:2218-2222.
[https://doi.org/10.1021/jf0305229]

- Jung MS, Lee GS and Chae HJ. (2004). In vitro biological activity assay of ethanol extract of radish. Journal of the Korean Society for Applied Biological Chemistry. 47:67-71.
-
Kahlon TS, Chapman MH and Smith GE. (2007). In vitro binding of bile acids by okra, beets, asparagus, eggplant, turnips, green beans, carrots, and cauliflower. Food Chemistry. 103:676-680.
[https://doi.org/10.1016/j.foodchem.2006.07.056]

- Kee JY, Kim MO, You IY, Chai JY, Hong ES, An SC, Kim H, Park SM, You n SJ and Chae HB. (2003). Effects of genetic polymorphisms of ethanol-metabolizing enzymes on alcohol drinking behaviors. Korean Journal of Hepatology. 9:89-97.
-
Kim JS. (2004). Effect of a alcohol detoxification beverage(ABD) contained Radix puerariae and Bambusae caules in Liquamen Phyllosachyos on the alcohol administered mouse. Journal of the Korean Society of Food Science and Nutrition. 33:318-323.
[https://doi.org/10.3746/jkfn.2004.33.2.318]

- Kim OK, Lee TG, Park YB, Park DC, Lee YW, Yeo SG, Kim IS, Park YH and Kim SB. (1996). Inhibition of xanthine oxidase by seaweed extracts. Journal of the Korean Society of Food Science and Nutrition. 25:1069-1073.
- Kwon SB, Kwon HJ, Jeon SJ, Seo HT, Kim HY, Lim JG and Park JS. (2020). Analysis of biological activities and functional components in different parts of asparagus. Korean Journal of Horticultural Science and Technology. 52:67-74.
-
Lee J, Hwang BH, Song HJ, Jang SH and Choe SY. (2016). Effect of black red ginseng mixture on alcohol metabolism in rats. Korean Journal of Food and Nutrition. 29:655-662.
[https://doi.org/10.9799/ksfan.2016.29.5.655]

-
Lee JW, Heo BG, Bae JH and Ku YK. (2015). Comparison of plant growth, dormancy breaking, yield, and biological activities of extracts in four asparagus cultivars. Korean Journal of Horticultural Science and Technology. 33:796-804.
[https://doi.org/10.7235/hort.2015.15080]

-
Lim AK, Jung MJ, Lee JW, Hong JH, Kim KS, Jung SB and Kim DI. (2011). Effect of alcohol detoxification beverage that contained Bulnesia sarmienti on alcohol-metabolizing enzymes and antioxidant enzyme activities. Korean Journal of Food Preservation. 18:407-413.
[https://doi.org/10.11002/kjfp.2011.18.3.407]

-
Maeda T, Honda K, Sonoda T, Motoki S, Inoue K, Suzuki T, Oosawa K and Suzuki M. (2010). Light condition influences rutin and polyphenol contents in asparagus spears in the mother-fern culture system during the summer-autumn harvest. Journal of the Japanese Society for Horticultural Science. 79:161-167.
[https://doi.org/10.2503/jjshs1.79.161]

-
Maeda T, Kakuta H, Sonoda T, Motoki S, Ueno R, Suzuki T and Oosawa K. (2005). Antioxidation capacities of extracts from green, purple, and white asparagus spears related to polyphenol concentration. HortScience. 40:1221-1224.
[https://doi.org/10.21273/HORTSCI.40.5.1221]

-
Martin-Belloso O and Llanos-Barriobero E. (2001). Proximate composition, minerals and vitamins in selected canned vegetables. European Food Research and Technology. 212:182-187.
[https://doi.org/10.1007/s002170000210]

-
Motoki S, Kitazawa H and Maeda T. (2012a). Improving the yield of the purple asparagus cultivar ‘Purple Passion’ by high density planting. Acta Horticulturae. 950:117-124.
[https://doi.org/10.17660/ActaHortic.2012.950.12]

-
Motoki S, Kitazawa H, Maeda T, Suzuki T, Chiji H, Nishihara E and Shinohara Y. (2012b). Effects of various asparagus production methods on rutin and protodioscin contents in spears and cladophylls. Bioscience, Biotechnology, and Biochemistry. 76:1047-1050.
[https://doi.org/10.1271/bbb.120143]

-
Nguyen MTT, Awale S , Tezuka Y, Le Tran Q, Watanabe H and Kadota S. (2004). Xanthine oxidase inhibitory activity of Vietnamese medicinal plants. Biological and Pharmaceutical Bulletin. 27:1414-1421.
[https://doi.org/10.1248/bpb.27.1414]

- Park M, Lee Y, Park H and Park J. (2016). An analysis of the supply and demand status of western vegetables and tasks. Korea Rural Economic Institute. Naju, Korea. R786. http://library.krei.re.kr/pyxis-api/1/digital-files/605ba745-b595-2a94-e054-b09928988b3c, (cited by 2021 Dec 8).
- Seong KC, Lee JS, Lee SG and Yoo BC. (2001). Comparison of growth characteristics by varieties and effects of rain shelter and mulching on the production of asparagus(Asparagus officinalis L.). Journal of Bio-Environment Control. 10:187-96.
-
Shin YJ, Hwang JM and Lee SC. (2013). Antioxidant and xanthine oxidase inhibitory activities of hot water extracts of medicinal herbs. Journal of the Korean Society of Food Science and Nutrition. 42:1712-1716.
[https://doi.org/10.3746/jkfn.2013.42.10.1712]

-
Stirpe F, Della Corte E and Lorenzoni E. (1969). The regulation of rat liver xanthine oxidase: Conversion in vitro of the enzyme activity from dehydrogenase(Type D) to oxidase(Type O). Journal of Biological Chemistry. 244:3855-3863.
[https://doi.org/10.1016/S0021-9258(17)36428-1]

-
Storch J and Ferber E. (1998). Detergent-amplified chemiluminescence of lucigenin for determination of superoxide anion production by NADPH oxidase and xanthine oxidase. Analytical Biochemistry. 169:262-267.
[https://doi.org/10.1016/0003-2697(88)90283-7]

-
Sullivan SA, Tran AQM, Xu G, Yin Y, Zhou C and Bae-Jump VL. (2017). Asparagus polysaccharide inhibits cell proliferation, adhesion and invasion in endometrial cancer cells. Gynecologic Oncology. 145:133-133.
[https://doi.org/10.1016/j.ygyno.2017.03.312]

-
Symes A, Shavandi A, Zhang H, Mohamed Ahmed IA, Al-Juhaimi FY and Bekhit AEDA. (2018). Antioxidant activities and caffeic acid content in New Zealand Asparagus(Asparagus officinalis) roots extracts. Antioxidants. 7:52. https://www.mdpi.com/2076-3921/7/4/52, (cited by 2021 Dec 8).
[https://doi.org/10.3390/antiox7040052]

-
Tsushida T, Suzuki M and Kurogi M. (1994). Evaluation of antioxidant activity of vegetable extracts and determination of some active compounds. Nippon Shokuhin Kogyo Gakkaishi. 41:611-618.
[https://doi.org/10.3136/nskkk1962.41.611]

-
Wang M, Tadmor Y, Wu QL, Chin CK, Garrison SA and Simon JE. (2003). Quantification of protodioscin and rutin in asparagus shoots by LC/MS and HPLC methods. Journal of Agricultural and Food Chemistry. 51:6132-6136.
[https://doi.org/10.1021/jf0344587]

-
Wang N, Zhang X, Wang S, Guo Q, Li Z, Liu H, et al. (2020). Structural characterisation and immunomodulatory activity of polysaccharides from white asparagus skin. Carbohydrate Polymer. 227:115314. https://www.sciencedirect.com/science/article/pii/S0144861719309816, (cited by 2021 Dec 8).
[https://doi.org/10.1016/j.carbpol.2019.115314]

-
Xiong D, Yu LX, Yan X, Guo C and Xiong Y. (2011). Effects of root and stem extracts of Asparagus cochinchinensis on biochemical indicators related to aging in the brain and liver of mice. American Journal of Chinese Medicine. 39:719-726.
[https://doi.org/10.1142/S0192415X11009159]

-
Zhang H, Brich J, Pei J, Ma ZF and Bekhit AED. (2019). Phytochemical compounds and biological activity in asparagus roots: A review. International Journal of Food Science and Technology. 54:966-977.
[https://doi.org/10.1111/ijfs.13993]