
Growth and Ginsenoside Content in Different Parts of Ginseng Sprouts Depending on Harvest Time
© The Korean Society of Medicinal Crop Science. All rights reserved.
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License ( http://creativecommons.org/licenses/by-nc/3.0 ) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Since the revised Ginseng Industrial Act was passed, ginseng sprouts have become a new medicinal vegetable for which there is high consumer demand. However, the existing amount of research and data on ginseng production has not kept pace with this changed reality.
In this study we analyzed the changes in the amounts of ginsenosides in different parts of growing ginseng sprouts during the period from when organic seedlings were planted in nursery soil until 8 weeks of cultivation had elapsed, which was when the leaves hardened. In the leaves, ginsenoside content increased 1.62 times with the panaxadiol (PD) system and 1.31 - 1.56 times with the panaxatriol (PT) system from 7 to 56 days after transplantation. During the same period, the total ginsenoside content of the stems decreased by 0.66 - 0.91 times, and those of the roots increased until the 21st day, and then underwent steep declines. The effect of fermented press cake extract (FPCE) and tap water (TP) on the total amount of ginsenoside per plant were similar, and could be represented with the equations y = 1.4330 + 0.2262x - 0.0008x2and y = 0.9555 + 0.2997x - 0.0031x2in which y = ginsenoside content x = amount of and on the total amounts of FPCE or TP, respectively after 26.4 days, however, the difference between ginsenoside content with FPCE and TP widened.
These results suggested that the amounts of ginsenosides in different parts of ginseng varied with the cultivation period and nutrient supply. These findings also provide fundamental data on the distribution of ginsenosides among plant parts for 2-year-old ginseng plants in the early- growth stage.
Keywords:
Panax Ginseng C. A. Meyer, Ginseng Sprouts, Ginsenoside, Panaxadiol, PanaxatriolINTRODUCTION
Ginseng (Panax Ginseng C. A. Meyer) is a perennial herbaceous plant belonging to the genus of Araliaceae that has been widely used for medicinal purposes in East Asia since 2,000 years ago.
Since a unique ingredient ginsenoside, plays a positive role, ginseng is known to have anticancer (Suh et al., 2007), anti-stress (Kim et al., 2003), neuro-protective (Lopez et al., 2007), and antidiabetic (Vuksan et al., 2008) effect.
As recent studies (Kim et al., 2010; Park, 2012) have found that the amount of ginsenosides is higher in leaves than roots, consumers and producers are interested in leaves of ginseng. As new farming method for ginseng growing sprouts, specifically, produces ginseng with the following procedure: seedlings that have passed organic or residual pesticide tests are harvested and stored frozen at −4℃.
Then, when there is a buying demand, the ginseng seedling is planted to grow sprouts for 2 to 8 weeks. Until 2014, hydroponic ginseng producers maintained pH and EC of the nursery soil at 6.0 ± 0.5 and 1.0 ± 0.2 dS/m for 3 to 4 months after transplanting the seedlings (RDA, 2014).
This common method had limitations in using leaves and stems as thatched vegetables. As the revised regulations of 2016 specified in the Ginseng Industrial Act (MAFRA, 2016) relaxed restrictions on nurturing ginsengs only with particular nutrient solutions except for the specified cultivation period, ginseng producers are able to use either nutrient solutions or clean water. As a result, ginseng leaves are now produced with a softer texture, and sales of domestically produced ginseng leaves in the vegetable market grew from KRW 600 million in 2014 to KRW 18.3 billion in 2016. This is an increase in market demand of approximately 30 times within two years and this continuous rapid growth seems to be positive.
With the revised Ginseng Industrial Act, ginseng sprouts have become a new medicinal vegetable with high consumer demand in the market. However, the existing research data on ginseng production does not reflect the changed reality; there are insufficient studies on ginseng sprouts, as the general study focus has been on hydroponics ginseng cultivated by the previous method that applied nutrient solutions to nursery soil or water for 3 to 4 months. In the case of 4-months-old hydroponics ginseng, the total amount of ginsenosides in stems and roots is 1.1 - 1.5%, while the leaves have 13.3 - 16.1%, which is 12 times greater than the amount in stems and roots (Kim et al., 2010). However, during the different stages of cultivation, the proportion of ginsenosides in ginseng changes (Shi et al., 2007).
Therefore, this study analyzed the changing amounts of ginsenosides in growing ginseng sprouts by parts during the period after organic seedlings are planted in nursery soil until eight weeks of cultivation, when the leaves harden.
This ginseng is either irrigated by fermented press cake extract (FPCE) or tap water (TW) during the 8-weeks of cultivation. This research analysis meets the current demand of the ginseng production market in addition to providing fundamental data on the distribution of ginsenosides by type for 2-years-old ginseng in the early-growth stage.
MATERIALS AND METHODS
1 Growth conditions of ginseng sprouts
For the analysis, this study sowed ginseng (Panax Ginseng C. A. Meyer) purchased from a farmhouse in March 2014 in nursery soil specifically mixed for ginseng production and these organic seeds were cultivated to organic seedlings of 0.8 g in a greenhouse protecting them from rain without agricultural pesticides and chemical fertilizers (Jang et al., 2015).
For long-term preservation, the seedlings were kept frozen at −4℃ after being harvested in the middle of November, and then in May of the following year the frozen seedlings were stored at 2 - 4℃ for five days and then replanted in 35-plug seedling trays.
The ginseng seedlings were moved to a closed cultivation system that maintained the temperature at 20 ± 1℃, relative humidity at 40 ± 10%, and CO2 level at 400 ± 50 μM (similar to natural atmosphere concentration). A consistent optical distance environment was set with 8 hours of LED lighting per day with a blending ratio of blue light and red light of 7 : 3 and photosynthetic photon flux of 50 ± 2 umol/m2/s with an LI-250A quantum sensor (LI-COR Co., Lincoln, NE, USA) on the leaf surfaces of 2-years-old ginseng at a distance of 20㎝ above them.
After setting up the environment, the soil moisture level was maintained at 15 - 18% by automatically controlling the space in groups with 500-times diluted FPCE and TW without any additional chemical nutrients. The fermented press cake in the FPCE composition was purchased from the market and the percentages of T-N, P2O5, K2O, CaO, MgO, and water content prior to dilution were 3.89, 2.21, 1.35, 0.89, 1.01 and 11%, respectively.
2 Nursery soil chemical analysis
This study analyzed chemical changes in soil composition after automatic applications of FPCE and TW every 4 weeks.
The assessments consisted of measuring changes in the pH and EC of the filtrate solution after drying the soil with wind and diluting it at 1 : 5 with distilled water and v/v (NIAST, 2012).
ACN analyzer (Vario Max CN, Hanau, Germany) was used to measure the total amounts of carbon and nitrogen and other organic matters were calculated based on the total carbon measurements.
An ICP-OES (GBC Scientific Equipment, Braeside, Australia) was used to measure the amount of P2O5 with the Lancaster method (Eo et al., 2011) and the amount of the exchangeable cation after being filtered with Toyo No. 5B.
3 Measurements of growth including chlorophyll content and dry weight
After replanting the ginseng seedlings, the chlorophyll content, leaf areas, and partial dry weights of 20 plants per treatment were measured every seven days.
The chlorophyll contents were measured by averaging the contents after using a portable chlorophyll meter (Konica Minolta, Tokyo, Japan) three times per ginseng and the leaf areas were measured with the WindDIAS image analysis system (ADC, London, England) to minimize the overlapping of the leaves.
The dry weights of ginseng parts were measured with an electronic scale (CAS, Seoul, Korea) after being parted into leaves, stem, and roots and dried in the same condition for five days at –80℃.
4 Analysis of partial ginsenosides
This study analyzed the amounts of 10 types of ginsenosides in the cultivated ginseng sprouts after categorizing them into leaves, stems, and roots.
First, 70% MeOH 2㎖ was added and mixed well with 0.2 g of the ginseng powder samples dried in the same condition and assessed with ultrasonic waves for 30 minutes at 50℃. Then the supernatant obtained by 15min centrifugation at 13,000 rpm was processed by Sep-Pak. In other words, a Sep-Pak Plus C18 cartridge was first conditioned by 3㎖ MeOH and then conditioned by 3㎖ dd-H2O as the second step. The ginsenoside component was extracted gradually by 2㎖ MeOH while the saccharide of the 1㎖ sample liquid was eliminated by 10㎖ dd-H2O loaded in a cartridge. The purified extract was filtered through a 0.45㎛ membrane filter and then used as an HPLC analysis sample (Jang et al., 2016).
The contents of ginsenosides were measured with the Nexera X2 UPLC system (Shimadzu, Kyoto, Japan) and 10 types of Rb1, Rb2, Rb3, Rc, Rd, Re, Rf, Rg1, Rg2, and Rh1 (ChromaDex, Irvine, CA, USA) were used as standard reagents.
The flow rate of the mobile phase (0 -1 min, 27% B; 1 - 6 min, 28% B; 6 - 10 min, 28% B; 10 - 30 min, 34% B; 30 - 33 min, 80% B and 33 - 35 min, 27% B) was 0.5 - 0.8㎖/min, the column temperature was 40℃, the wavelength of the UV detector was 203㎚, and the column was Halo RP-amide (4.6 × 150㎜, 2.7㎛, Advanced Analytical Technologies Inc., Wilmington, DE, USA).
5 Statistical analysis
The results obtained from this experiment on FPCE and TW treatment plot were analyzed with student’s t-test using the SAS software package (SAS v9.4, SAS Institute Inc., Cary, NC, USA), and the difference was proven to be statistically significant when the p = value was under 0.05.
RESULTS AND DISCUSSION
1 Changes in nursery soil chemical properties
Table 1 shows the changes in nursery soil chemical properties when the FPCE and TW were automatically adjusted to 15 - 18% moisture content during the cultivation of ginseng (Panax Ginseng C. A. Meyer) sprouts.
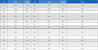
Changes of nursery soil chemical properties by auto-irrigation of fermented press cake extract and tap water during cultivation period.
There was no statistical difference between FPCE and TW in pH, OM, and Na regardless of the treatment days and in Ca after 56 days of treatment. However, EC, NO3, P2O5, K, and Mg were measured higher in FPCE than in TW and when FPCE was used, the amounts of these components were in an increasing trend prior to cultivation to 56 days of cultivation. When TW was used, EC, NO3, P2O5, K, and Mg tended to decrease or stay the same except Ca. This seems to be the case because the components were filtered as extracts by the water without any nutrition added. (Jang et al., 2013).
The continuous watering maintained the moisture level and increased the pH compared to the level prior to cultivation, but the pH ranged around at 5.4 - 6.2, the appropriate level for non-soil cultivation. When the level of pH increases beyond the appropriate level in non-soil cultivation based on peat- moss rather than nursery soil with minerals, the soil strongly adheres with inorganic substance (Lucas and Davis, 1961; Park et al., 2014). This prevents crops from absorbing necessary nutrients so that non-soil cultivation prompts various considerations for growing ginseng sprouts.
2 Effect of FPCE and TW on growth by harvest time
Differences in nutrient compositions significantly influence the parts of ginseng sprouts.
Seven days after replanting seedlings, the root growth of ginseng sprouts produced by FPCE decreased, while the stems increased and leaves had no difference. In terms of the total dry weights of ginseng sprouts, the average weights were not significantly different between TW (148.8㎎/g) and FPCE (148.3㎎/g).
This means that the distribution of matter production started from the rhizome of the ginseng as the emergence of the ground part is done first with the stem rather than with the leaves (Lee et al., 1996; Proctor, 2008). Nonetheless, but total amount did not change during cultivation. This growth pattern continued until the 14th day of cultivation when the weight of leaves became greater, and the weight difference between the stem and leaves widened until the 28th day. From the 35th to the 42nd day, the difference narrowed but was greater than the error range. Then, after the 42nd day, it was clear that ginseng sprouts grown with FPCE were greater in dry weight than the ones cultivated with TW.Fig. 1

Partial dry weight of ginseng sprouts applied fermented press cake extract (FPCE) and tap water (TW) after transplanting seedling.A; leaf, B; stem, C; root. The vertical error bars represent the standard errors (n = 20). *p < 0.05 and **p < 0.01 compared to FPCE and TW. NS; Not significant.
Regardless of the nutrient supply, the growth of the top shoots rapidly increased until the 35th day, similar to the weight of the roots. Then after the 35th day, root enlargement became more apparent. The deficiency of N, K, and Mg hinders the growth of ginseng leaves.
In particular the deficiency of N, as the plant cell components of amino acids, protein, and nucleic acids directly inhibit the growth of plant leaves. If the deficiency persists, most plant species exhibit yellow leaves, especially old leaves close to the base (Kwon et al., 1999; Lee et al., 2013; Kim et al., 2015).
The SPAD value in Fig. 2 shows that, despite the chlorophyll-producing ability of the seedling germ, damage to the leaves occurs when the inorganic element supply is not smooth from the 35th day.

Leaf area and SPAD value of ginseng sprouts applied fermented press cake extract (FPCE) and tap water (TW) after transplanting seedling.The vertical error bars represent the standard errors (n = 20). *p < 0.05 and **p < 0.01 compared to FPCE and TW. NS; Not significant.
Plants that have the N deficiency often have stems with a woody texture. This lignification is due to the accumulation of carbohydrates that are not used in the synthesis of amino acids or other N-containing compounds (Bloom et al., 1993; Aber et al., 2003) K, present in the plant in the form of cation, plays an important role in regulating the osmotic potential of plant cells. The stem of a K-deficient plant is thin and weak (Navetiyal et al., 1989). In this experiment to produce ginseng sprouts, it is considered that N and K deficits influenced the dry weights of the stems at the same time from an early stage.
In plant cells, Mg also plays a specific role in the activation of enzymes involved in respiration, photosynthesis, DNA and RNA synthesis. In the experiment, since Mg is also part of the chlorophyll ring structure (Wahome et al., 2001), it would have been deficient with N, which would have inhibited the expansion of ginseng leaves and maintenance of chlorophyll content. However, on the 21st day, while the ginseng sprouts with TW applied showed such deficient conditions that nutrient production was not active at full capacity, they had a 4.4% greater mass in average total weight than the ones with FPCE with 19.6% lower mass for the upper parts and 30.7% greater root weights.
This is because the distribution of matter production originating from the root can consume more inherent stored matter for producing leaves or stems. This suggests that in terms of per plant weight, it is more beneficial for farms with difficulties in applying additional nutrients to harvest ginseng sprouts only on water on the 21st day after planting ginseng seedlings.
3 Analysis of partial ginsenoside during cultivation period
Changes in the ginsenoside contents of ginseng sprouts during automatic applications of FPCE and TW are shown in Fig. 3. As existing studies found, leaves have 6 - 8 times greater total ginsenoside contents than ones in roots or stems (Kim et al., 2010).

Changes in partial ginsenoside contents of ginseng sprouts applied fermented press cake extract (FPCE) and tap water (TW) after transplanting seedling.A; leaf, B; stem, C; root. Panaxadiol (PD) = Rb1+Rb2+Rb3+Rc + Rd, panaxatriol (PT) = Re+Rf + Rg1+Rg2+Rh1. Data represent the means ± SD (n = 3).
The ginsenoside contents of roots and stems were similar in total, but the composition ratio differences of the panaxadiol (PD) system (Rb1, Rb2, Rb3, Rc and Rd) and panaxatriol (PT) system (Re, Rf, Rg1, Rg2 and Rh1) were large. In the case of roots, the ratio of PD and PT is about 1 : 1, but stems is about 1 : 8.Fig. 4

Changes in partial total ginsenoside per plant of ginseng sprouts applied fermented press cake extract (FPCE) and tap water (TW) after transplanting seedling.A; leaf, B; stem, C; root. Data represent the means ± SD (n = 3).
While most of the ginsenosides were composed of PT, P6H and chtochrome P450 genes are activated more in roots than other parts. This seems to be the case because protopanaxadiol can easily be converted to protopanaxatriol (Huang and Zhong, 2013).
Although the total ginsenoside content of ginseng is important, the PD/PT ratio is a more important factor in terms of its efficacy to consumers (Jin et al., 1999). PDbased ginsenoside is effective for central nervous system sedation and anti-inflammation, and PT ginsenoside is effective for fatigue recovery, strength improvement, and cholesterol reduction (Kim et al., 2008).
Thus, the optimal ratio seems to be approximately 1.0. According to Kim et al. (2010) the ratio of PD/PT in hydroponic ginseng roots is 0.5 - 0.6, but in this study, in the case of ginseng sprouts, they have a PD/PT ratio of approximately 0.8 - 1.0, which is a level similar to 4-yearsold ginseng (Han et al., 2013).
The composition of ginsenoside contents in ginseng sprouts showed various patterns by part with varying cultivation periods. In the case of leaves, ginsenoside contents increased 1.62 times in the PD system and 1.31 - 1.56 times in the PT system from 7 days to 56 days after transplantation. In the case of stems, the ginsenoside contents decreased by 0.6 - 0.89 times in the PT system, while the PT system contained the majority of the ginsenosides, and the total similarly decreased by 0.66 - 0.91 times.
The ginsenoside contents in roots increased until the 21st day; and then showed a steep decline, which seems to be related to the distribution of the matter substances in the roots. This assumption is based on the observation that as the root dry weight decreased, the intrinsic ginsenoside contents increased and then decreased again from the 21st day, while the dry weight increased.
Similarly, ginsenoside contents in leaves tended to increase gradually after the 28th day, when the dry weight of leaves started to be maintained.
Many studies comparing ginsenoside contents by ginseng age have shown that the ginsenoside concentrations of 4- years-old ginseng was higher than that of 6-years-old ginseng (Court et al., 1996; Li and Wardle, 2002).
This indicates that there is a significant relationship between ginsenoside contents and ginseng growth. In particular, the difference is more apparent among shortterm lived ginseng sprouts.
This study consistently found that ginsenoside contents was higher with TW-applied ginseng sprouts which had less leaf growth. However, as there was no significant growth difference in stems or roots by FPCE and TW, the more apparent difference came from the number of FPCE applications.
Ginsenoside is a glycoside conjugated with aglycone, the chemical structure of which is a dammarane triterpene, and is largely influenced by the accumulation of photosynthetic products. Excessive light uptake and accumulation of Na contents cause stress in plants, thus inhibiting the growth of ginseng and interfering with the accumulation of carbohydrates (Ashraf and Orooj, 2006; Jang et al., 2016). Ginseng, which has fewer accumulated saccharides, is forced to increase its ginsenoside content naturally. The ginsenoside changes per plant in leaf + stem + root were FPCE (y = 1.4330 + 0.2262x - 0.0008x2) and TW (y = 0.9555 + 0.2997x - 0.0031x2), where the x-axis is the period and the y-axis is the amount of ginsenosides.
Analysis based on day 26.4, when the two lines intersect, showed that the effects of FPCE and TW on the amounts of ginsenosides were similar, and then after day 26.4, the difference widened.
As ginsenosides are more abundant and the rate of ginsenoside accumulation is faster on leaves than on roots or stems, the study results show that the effects of FPCE and TW on ginseng ginsenosides are similar in leaves.
ACKNOWLEDGMENTS
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries(IPET) through Advanced Production Technology Development Program(315095-04-HD030), funded by Ministry of Agriculture, Food and Rural Affairs(MAFRA).
References
-
JD Aber, CL Goodale, SV Ollinger, ML Smith, AH Magill, ME Martin, RA Hallett, JL Stoddard, Is nitrogen deposition altering the nitrogen status of northeastern forests?, Bioscience, (2003), 53, p375-389.
[https://doi.org/10.1641/0006-3568(2003)053[0375:indatn]2.0.co;2]

-
M Ashraf, A Orooj, Salt stress effects on growth, ion accumulation and seed oil concentration in an arid zone traditional medicinal plant ajwain(Trachyspermum ammi [L]. Sprague)., J. Arid Environ, (2006), 64, p209-220.
[https://doi.org/10.1016/j.jaridenv.2005.04.015]

-
AJ Bloom, LE Jackson, DR Smart, Root growth as a function of ammonium and nitrate in the root zone., Plant Cell Environ, (1993), 16, p199-206.
[https://doi.org/10.1111/j.1365-3040.1993.tb00861.x]

-
WA Court, LB Reynolds, JG Hendel, Influence of root age on the concentration of ginsenosides of American ginseng(Panax quinquefolium)., Can. J. Plant Sci, (1996), 76, p853-855.
[https://doi.org/10.4141/cjps96-144]

-
J Eo, KC Park, BR Yeon, Changes in soil biota affected by the application of organic materials in reclaimed upland and paddy-converted soils cultivated with Korea ginseng., Korean Journal of Soil Science and Fertilizer, (2011), 44, p872-877.
[https://doi.org/10.7745/kjssf.2011.44.5.872]

-
JS Han, HS Tak, GS Lee, JS Kim, RJ Woo, JE Choi, Comparison of ginsenoside content and ratio of root tissue according to root age and diameter in Panax ginseng C. A. Meyer., Korean Journal of Medicinal Crop Science, (2013), 21, p342-347.
[https://doi.org/10.7783/kjmcs.2013.21.5.342]

-
C Huang, JJ Zhong, Elicitation of ginsenoside biosynthesis in cell cultures of Panax ginseng by vanadate., Process Biochem, (2013), 48, p1227-1234.
[https://doi.org/10.1016/j.procbio.2013.05.019]

-
IB Jang, DY Hyun, SW Lee, YC Kim, JU Kim, GC Park, KH Bang, GH Kim, Analysis of growth characteristics and physiological disorder of Korean ginseng affected by application of manure in paddy-converted field., Korean Journal of Medicinal Crop Science, (2013), 21, p380-387.
[https://doi.org/10.7783/kjmcs.2013.21.5.380]

-
IB Jang, DY Lee, J Yu, HW Park, HS Mo, KC Park, DY Hyun, EH Lee, KH Kim, CS Oh, Photosynthesis rates, growth, and ginsenoside contents of 2-yr-old Panaxginseng grown at different light transmission rates in a greenhouse., J. Ginseng Res, (2015), 39, p345-353.
[https://doi.org/10.1016/j.jgr.2015.03.007]

-
IB Jang, J Yu, KB Kweon, SJ Suh, Effect of controlled light environment on the growth and ginsenoside content of panax ginseng C. A. Meyer., Korean Journal of Medicinal Crop Science, (2016), 24, p277-283.
[https://doi.org/10.7783/kjmcs.2016.24.4.277]

-
SH Jin, JK Park, KY Nam, SN Park, NP Jung, Korean red ginseng saponins with low ratios of protopanaxadiol and protopanaxatriol saponin improve scopolamine-induced learning disability and spatial working memory in mice., J. Ethnopharmacol, (1999), 66, p123-129.
[https://doi.org/10.1016/s0378-8741(98)00190-1]

-
DH Kim, YS Moon, TH Lee, JS Jung, HW Suh, DK Song, The inhibitory effect of ginseng saponins on the stressinduced plasma interleukin-6 level in mice., Neurosci. Lett, (2003), 353, p13-16.
[https://doi.org/10.1016/j.neulet.2003.08.070]

- GS Kim, DY Jyun, YO Kim, SW Lee, YC Kim, SE Lee, YD Son, MJ Lee, CB Park, HK Park, SW Cha, KS Song, Extraction and preprocessing methods for ginsenosides analysis of Panax ginseng C. A. Meyer. Korean Journal ofMedicinal., Crop Sci, (2008), 16, p446-454.
- GS Kim, DY Hyun, YO Kim, SE Lee, H Kwon, SW Cha, CB Park, YB Kim, Investigation of ginsenosides in different parts of Panax ginseng cultured by hydroponics., Korean Journal of Horticultural Science and Technology, (2010), 28, p216-226.
-
JU Kim, DY Hyun, YC Kim, JW Lee, IC Jo, DH Kim, KH Kim, JK Shon, Effects of salt in soil condition on chlorophyll fluorescence and physiological disorder in Panax ginseng C. A. Meyer., Korean Journal of Medicinal Crop Science, (2015), 23, p446-453.
[https://doi.org/10.7783/kjmcs.2015.23.6.446]

- TR Kwon, PJ.C Harris, WF Bourne, Partitioning of Na+, K+, proline, and total soluble sugar in relation to the salinity tolerance of Brassica juncea and Brassica rapa., Journal of the Korean Society for Horticultural Science, (1999), 40, p425-430.
- JH Lee, MG Lee, KT Chio, SS Lee, Identification of age of cultivated ginseng(Panax ginseng C. A. Meyer) basedon stem vestige of rhizome., Korean Journal of Ginseng Science, (1996), 20, p72-77.
-
SW Lee, KC Park, SH Lee, JM Park, IB Jang, KH Kim, Soil chemical property and leaf mineral nutrient of ginseng cultivated in paddy field occurring leaf discoloration., Korean Journal of Medicinal Crop Science, (2013), 21, p289-295.
[https://doi.org/10.7783/kjmcs.2013.21.4.289]

- TS.C Li, D Wardle, Seasonal fluctuations of leaf and root weight and ginsenoside contents of 2-, 3-, and 4-year-old American ginseng plants., Horttechnology, (2002), 12, p229-232.
- MV.N LA3pez, PG.S Cuadrado, OM.P Ruiz-Poveda, AM.V.D Fresno, EC Accame, Neuroprotective effect of individual ginsenosides on astrocytes primary culture., Biochim. Biophys. Acta, (2007), 1770, p1308-1316.
-
RE Lucas, JF Davis, Relationships between pH values of organic soils and availabilities of 12 plant nutrients., Soil Sci, (1961), 92, p177-182.
[https://doi.org/10.1097/00010694-196109000-00005]

- MAFRA, Enforcement regulation of Korean ginseng industry act. Article 8(Tillage method and guidance). Ministry of Agriculture, Food and Rural Affairs. Sejong, Korea. p.169-175, (2016).
- NIAST, (2012), Methods of soil chemical analysis. National Institute of Agricultural Sciences and Technology. Rural Development Adminstration. Suwon, Korea. p.16-770.
- RC Navetiyal, V Ravindra, YC Joshi, Germination and early seedling growth of some groundnut(Arachis hypogaea L.) cultivars under salt stress., J. Plant Physiol, (1989), 32, p251-253.
-
HW Park, IB Jang, YC Kim, HS Mo, KC Park, J Yu, JU Kim, EH Lee, KH Kim, DY Hyun, Growth characteristics of ginseng seedlings as affected by mixed nursery soil under polyethylene film covered greenhouse., Korean Journal of Medicinal Crop Science, (2014), 22, p363-368.
[https://doi.org/10.7783/kjmcs.2014.22.5.363]

- JH Park, Antioxidant activities in shoots and roots of hydroponic cultured ginseng., Journal of Applied Oriental Medicine, (2012), 16, p21-26.
- JT.A Proctor, Source-sink relations in North American ginseng seedlings as influenced by leaflet removal., J. Ginseng Res, (2008), 32, p337-340.
- RDA, Ginseng, Eumseong, Korea, Rural Development Adminstration, (2014), p90-246.
-
W Shi, Y Wang, J Li, H Zhang, L Ding, Investigation of ginsenosides in different part and ages of Panax ginseng., Food Chem, (2007), 102, p664-668.
[https://doi.org/10.1016/j.foodchem.2006.05.053]

- SO Suh, YJ Boo, JM Park, J Kim, Prospective study for Korean red ginseng extract as an immune modulator following a curative surgery in patients with advanced colon cancer., J. Ginseng Res, (2007), 31, p54-59.
- V Vuksan, MK Sung, JL Sievenpiper, PM Stavro, AL Jenkins, MD Buono, KS Lee, LA Leiter, KY Nam, JT Arnason, M Choi, A Naeem, Korean red ginseng(Panax ginseng) improves glucose and insulin regulation in wellcontrolled, type 2 diabetes: Results of a randomized, doubleblind, placebo-controlled study of efficacy and safety., Nutr. Metab. Cardiovasc. Dis, (2008), 18, p46-56.
- PK Wahome, HH Jesch, I Pinker, Effect of sodium chloride on Rosa plants growing in vitro., Sci. Hortic. (Amsterdam), (2001), 90, p187-191.