
Physicochemical Composition and Properties of Sancho Oil Extracted from Zanthoxylum schinifolium Seeds
 ; Seung Mi Kang2
; Seung Mi Kang2 ; Seong Hyeon Yong3
; Seong Hyeon Yong3 ; Do Hyun Kim4 ; Kwan Been Park5 ; Myung Suk Choi6, 7, †
; Do Hyun Kim4 ; Kwan Been Park5 ; Myung Suk Choi6, 7, †
This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The seed oil of Zanthoxylum schinifolium S. et Z. (sancho) is a traditional vegetable oil that is usually sold at high prices. However, there is no information on the characteristics of sancho oil according to the collection site in Korea. Therefore, this study investigated characteristics such as the fatty acid composition, acid value, peroxide value, color, consistency, volatile compound composition, and antioxidant activity of sancho oil from different sites.
Sancho seeds were collected from four sites (Hadong, Gapyeong, Jinan, and Yeongwol), that produce sancho oil, which was extracted by pressing. The content of major fatty acids, acid value, peroxidation degree, color and antioxidant activity were significantly different between the by collection sites. However, there was no significant difference in viscosity.
The fatty acid content of sancho oil varied according to the collection site, and properties such as acid value and antioxidant activity were different. Therefore, it is necessary to standardize the production and distribution of sancho oil produced in Korea, and studies to identify high-yielding varieties are important.
Keywords:
Zanthoxylum schinifolium, Acid Value, Antioxidant, Collection Site, Fatty Acid, Sancho OilINTRODUCTION
As the national income increases, the production and consumption of edible oils and fats, and processed products are rapidly increasing every year (Shin, 2017). In particular, Korea is very dependent on imports as the supply is very insufficient compared to the consumption of oil (Yang et al., 1996). In addition, until now, edible oils and fats were mainly supplied from crops, but most of them have been used for food preparation (Shin, 2017). However, it is known that plant-derived oils and fats have various physiological activities and the function of cooking food.
Vegetable oil resources used for food so far are camellia oil (Yang et al., 1996), peach and apricot seed oil (Park et al., 1984), pine oil (Kim and Yoon, 1975), and evening primrose oil (Pyo and Ahn, 1989) and others. However, these vegetable oils and fats are still in a state of insufficient development, so it is necessary to actively develop them to increase income in rural areas and improve people's health and welfare.
Sancho (Zanthoxylum chinifolium S. et Z.) is one of the spices before using red pepper and has been widely used in Gangwon-do for extraction fat using fruits, for powder spices or pickles, and for medicinal and oil refining (Jeong and Shin, 1990). About 200 species are distributed around the world, and they grow in mountainous areas. The height of the tree is 3 m, the twigs are thorny, and they are reddish-brown. The fruits are capsules, 4 ㎜ long, greenish-brown, and split into 3 pieces when ripe, and black seeds come out. Sancho trees use leaves, bark, and fruits, which contain sour taste, essential oil, and fats and oils.
Sancho has aromatic, anti-inflammatory, and diuretic properties and is used as an anthelmintic, respiratory disease, and antiseptic. In addition, it has detoxification, and appetite enhancement, toothache, neuralgia, poor circulation, cold, paralysis treatment, etc. is very diverse (Lee, 1996; Kim, 2003; Lim et al., 2003). In addition, sanchoo oil has been reported to have antibacterial activity (Seo et al., 1999; Park et al., 2008; Kim et al., 2020), insecticidal effect (Choochote et al., 2007), and anti-inflammatory effect (Lee et al., 2009). However, most of these studies are limited to identifying the biological activities of the bark, leaves, and fruits of the sancho tree, so the research on sancho oil is very insignificant.
Recently, with the improvement of the income level, interest in vegetable oil resources with biological activity is increasing, and the demand is also rapidly growing. However, there are few studies on domestic oil resources (Kim et al., 2017). In particular, because of difficult growth, there is no community formation and no selection of excellent individuals. Therefore, it is necessary to establish basic data on domestic oil resources such as fatty acid content and oil characteristics.
Genetic and environmental factors determine plant yield, protein and oil concentrations (Wolf et al., 1982; Maestri et al., 1998). In particular, it is known that unsaturated fatty acids are greatly affected by environmental conditions such as weather and soil (Wolf et al., 1982).
Vegetable oils and fats such as sancho oil have high acidity and lack quality control from production to distribution compared to edible oils. Vegetable oils have limited usefulness due to changes that occur during processing, cooking and storage (Kim et al., 2020). Changes such as the oxidation of oils and fats can cause severe problems in food hygiene by generating harmful substances (Song et al., 2003). Although sanchoo oil has been used in folk remedies for a long time, there may be problems in distribution and use as the series of processes from extraction, use, and storage are not scientifically identified. In this study, the oil content, oil properties, and antioxidant activity of sancho oil extracted in four collection sites of Korea were investigated.
MATERIALS AND METHODS
1. Material
The Sancho (Zanthoxylum chinifolium S. et Z.) seeds used in the experiment were collected from Hadong (H), Gapyeong (G), Jinan (J), and Yeongwol (Y) fields, which are major production areas of sancho oil. Seeds were collected from Buchun-ri, Hwagae-myeon, Hadong-gun, Gyeongsangnam-do (35°16'9.32"N, 127°64'9.61"E), Igok-ri, Buk-myeon, Gapyeong-gun, Gyeonggi-do (37°88'0.19"E, 127°53'4.81"E), Sammok Yeongwol-eup, Yeongwol-gun, Gangwon-do (37°21'5.69"N, 128°50'1.81"E), Jinan-eup, Jinan-gun, Guryong-ri (35°78'01.60"N, 127°44'88.53"E), Jeollabuk-do.
Seeds were collected on September 25 - 30, and the condition of the seeds was ripe. For extraction sanchoo oil, 5 plants of each collection site were collected and mixed for extraction. All organic solvents used in the experiment were of extra grade.
2. Extraction of sancho oil
The collected seeds were selected immediately, and the oil was extracted within 3 days of collection. The extraction of Sansho oil was performed by the compression method. After picking the sancho seeds, they were extracted with an extraction machine (Gaebangagan, Oscarelectronic Co., Ltd., Gimhae, Korea). The oil was cooled at room temperature and centrifuged at 2,500 rpm for 5 minutes using a centrifuge. The supernatant was collected and stored at 5℃ and used as a sample.
3. The prepataion process for fatty acids analysis in sancho oil
Analysis of the fatty acid content of sancho oil was performed with a slight modification to the method of Lee (1998).
0.5 N sodium hydroxide (NaOH) and methanol (CH3OH) solution was added to sancho oil, reacted in a water bath at 100℃, boron trifluoride methanol (BF3·MeOH) solution 14% in methanol was added, and the reactant was mixed with nucleic acid, hydrolyzed and cooled to room temperature.
The cooled hydrolyzate is mixed with a saturated saline layer and stirred, then the nucleic acid layer is separated and put in an airtight container, then anhydrous sodium sulfate is added and left for 24 hours, diluted 100 times in ethyl ether, filtered with a membrane filter (40 ㎛).
4. Analysis precess of fatty acids in sancho oil
DV-WAX (60 m × 0.25 ㎛ × 0.25 ㎛) was used as the analytical column, nitrogen gas was used as the mobile phase, and the speed of the mobile phase was 1.3 ㎖/min. The oven temperature is held at 150℃ for 1 minute, then heated to 180℃ at 20℃/min for 2 minutes, then raised to 3℃/min to 230℃ for 5 minutes, and then held for 5 minutes. Heat to 250℃ at 5℃ per minute for a while. The temperature of the injector and detector was maintained at 250℃. The fatty acid standard used and the search formula is shown in Table 1.
5. Volatile compound of sancho oil
The sancho oil stored at 5℃ was left at room temperature for 1 hour, shaken in a homogeneous state, diluted 1 : 1 with sancho oil and ethyl ether, filtered with a 40 ㎛ membrane filter, and then GC-MS (Agilent GC 5975C, Agilent Technologies Inc., Santa Clara, CA, USA).
The analysis column (Agilent 19091s-433HP-5MS, Agilent Technologies Inc., Santa Clara, CA, USA) and nitrogen gas was used as the mobile phase. Helium was used as a carrier gas at a rate of 1 ㎖/min, and 1 ㎕ of sancho oil was injected. The effluent of the GC column was introduced directly into the source of the MS. Spectra were obtained in the EI monde with 70 eV ionization energy. The sector mass analyzer was set to scan from 50 to 800 amu for 2 s. The identification was carried out by comparison of mass spectra with those in the mass spectra library (The Wiley Registry of Mass Spectral Data, 6th ed.). Contents of volatile compounds were determined through relatively area (%) on analyzed peaks.
The coefficient of determination (R2) for the calibration curve was 0.99 or more, and the instrument detection limit of the GC for volatile substances was measured after repeating 3 repeated analyzes of the minimum concentration standard solution, and the instrument detection limit was 0.13 - 0.41, The relative standard deviation was 0.01 - 0.58, and the accuracy was 90.1% - 105.3%.
6. Acid value measurement of sancho oil
The acid value of sancho oil and blending oil was measured according to American Oil Chemists` Society (AOCS, 1990). 1.0 g of the sample was taken in an Erlenmeyer flask and mixed with 20 ㎖ of 1 : 2 (v/v) Ethanol (Sigma-Aldrich Co., St. Louis, MO, USA) was added to dissolve the oil.
The phenolphthalein solution (Sigma-Aldrich Co., St. Louis, MO, USA) was used as an indicator and titrated with 0.1 N potassium hydroxide (KOH) (Sigma-Aldrich Co., St. Louis, MO, USA) until the ethanol solution became pale red. The following equation determined the acid value, and the experiment was repeated three times. The acidity was measured in an incubator (SH-75B, Seyoung Scientific Co., Bucheon, Korea) adjusted to 25℃.
- A: volume of 0.1 N KOH (㎖), F: tilter of 0.1 N KOH (㎖)
7. Peroxide value measurement
The peroxide value of sancho oil was measured by the method of AOAC (1984).
In a 250 ㎖ Erlenmeyer flask, add 2 g of sancho oil to the correct amount using an electronic balance, add a mixed solution of acetic acid : chloroform (3 : 2, v/v) to dissolve sancho oil, add 1 ㎖ of saturated potassium iodide, and mix well. Then, it was reacted in a dark place for 10 minutes. 30 ㎖ of distilled water and 1 ㎖ of the starch indicator were added to the reaction product to give a reaction color (blue). Then, 0.01 N Na2S2O3 solution was added with a burette and titrated until colorless. A blank experiment was conducted to determine the zero point of the peroxidation value, and then the peroxidation value was measured by the following formula.
- v1: titrated amount of 0.01 N Na2S2O3 solution (㎖)
- v2: Blank test titration of 0.01 N Na2S2O3 solution (㎖)
- f: concentration titer of 0.01 N Na2S2O3 solution
- s: amount of sample taken (g)
8. Chromaticity analysis
For chromaticity analysis, the sancho oil was thawed at 20℃ for 30 minutes, homogeneously mixed, and analyzed with a colorimeter (Spectra Magic NX, Konica Minolta, Tokyo, Japan) to obtain a Lab value.
9. Viscosity analysis
Using a viscometer (Viscometer, DV-II +PRO, Brookfield, Toronto, Canada), 100 ㎖ of sancho oil was taken, and the temperature was automatically set at 20℃ at 100 rpm for 30 seconds, and then the viscosity was measured.
10. Statistical analysis
All experiments were performed 3 times. Data are presented as means and standard deviation and analyzed by One-way ANOVA using the IBM SPSS statistical package (Ver. 24, IBM Co., Armonk, NY, USA). Means were compared at 5% significance level using Duncan's Multiple Range Test (DMRT) comparison (p < 0. 05).
RESULTS AND DISCUSSION
1. Oil content by collection site
The content of sancho (Zanthoxylum chinifolium S. et Z.) oil extracted from sancho seeds did not differ significantly between collection sites (Fig. 1).

Content of sancho oil collected from Hadong (H), Gapyung (G), Jinan (J) and Youngwol (Y).The seeds of each of the five plants were collected from September 25 to 30, when the seeds were ripe, and the seeds were mixed to extract the oil and then quantified. No statistical significance (p > 0.05).
The content of vegetable oil mainly belongs to quantitative traits and is influenced by various environments during the growth period (Lee, 1988). Factors involved in the heritability of edible oil resources such as rapeseed and sesame seeds have been reported as flowering and maturation phases.
The reason why there was no significant difference in oil content between collection sites in this study may be due to either not genetically differentiated sancho trees or the extraction of three sancho seeds by combining them. Also, in this result, it seems that variation between individuals is more important than the variation between collection siteal communities. However, it is judged that future studies such as the selection of high-quality individuals are needed.
2. Fatty acid content and proportion in oil
The content of major fatty acids and total fatty acids was different for each collection site of sancho oil (Table 2). The highest content of major fatty acids was 270 g/ℓ in Yeongwol sancho oil, and the lowest content was 240 g/ℓ in Hadong sancho oil.
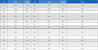
Saturated and unsaturated fatty acids content of sancho oil collected from Hadong (H), Gapyung (G), Jinan (J) and Youngwol (Y).
The content ratio of major fatty acids in sancho oil also showed a significant difference. The content of myristic acid, a long-chain fatty acid with an even number of carbons (Verruk et al., 2019), was highest in Jinan (0.42 g/ℓ). The remaining three regions were similar (0.22 - 0.17 g/ℓ). Palmitic acid content was highest in Yeongwol (41.51 g/ℓ) and least in Gapyung (34.04 g/ℓ).
In the case of palmitoleic acid, Hadong (9.57) and Gapyeong (9.24) were high, Jinan (7.27) and Yeongwol (7.06) were relatively low. The stearic acid content by collection regions was in the order of Jinan (9.80), Yeongwol (9.04), Gapyeong (5.45) and Hadong (4.11). The content of oleic acid by collection site was highest in Jinan (85.52) and least in Yeongwol (75.26). On the other hand, linoleic acid was measured in Yeongwol, Gapyeong, Jinan, and Hadong sancho oil in that order, with 77.89 g, 70.85 g, 69.14 g, and 62.92 g per liter, respectively. In the case of linolenic acid contents, Yeongwol was highest in the collection regions and was 59.48 g.
It is known that the fatty acid ratio of sancho oil varies significantly between cultivars. In the case of rapeseed oil, The ratio of omega-6/omega-3 fatty acids was 2.20 - 3.68 with the highest in Hanla and lowest in Naehan, There were differences between the cultivars (Lee et al., 2014). Also, when the fatty acids of Korean soybean genetic resources were measured, there were differences between varieties (Choung, 2006). This result is an important characteristic that can show the difference between varieties, and it can be used to suggest the possibility of breeding varieties with high specific components required.
In addition, in the case of sancho oil, the content of unsaturated fatty acids is high, and these numerical differences appear differently depending on the species. In the case of camellia oil, the high fatty acid content was oleic acid, which accounted for about 84.7% of the total fatty acid (Yang et al., 1996). In the case of soybean oil, a representative edible oil, the content of linoleic acid among 86.2% of the unsaturated fatty acids was 54.6% (Shin and Kim, 1982). These differences seem to be a unique characteristic between species, and the high content of unsaturated fatty acids can suggest the possibility as a functional material and the maintenance function.
3. Types and content of volatile compounds in sancho oil
The content of volatile compounds in sancho oil extracted from seeds collected from 4 collection sites was investigated (Table 3). Volatile compounds of sancho oil did not show significant differences among 4 collection sites. About 10 types of volatile compounds were detected in sancho oil. Sancho oil between production areas contained terpenoids and phenylpropene. The monoterpene content was different in each region, but it was contained in a small amount, less than 1%. Among them, as monoterpenes, ocimene, caryophcaryophyllene, humulene, germacrene, and spathulenol were contained, and the content was less than 1%.
The types and numbers of volatile compounds in this study were different from other reports. Chang and Kim (2008) reported that 60 types of turpentine were detected as a result of analyzing the oil in Z. schinifolium by G C-MS. In the seed-derived oil, the main compounds were β-phellandrene and citronella. Bae et al. (2011) also found 46 essential oil components in the fruit of Z. schinifolium, and the main components were α-pinene, β-myrcene, β-ocimene, 2-nonanone, estragole, 2-undecanone, and β-caryophyllene.
In addition, 10 types of volatile compounds were detected in this study, and the number of detected compounds was significantly lower than in other reports. The biggest reason is thought to be due to the extraction method. Chang and Kim (2008) extracted essential oil by the steam distillation method, and Bae et al. (2011) extracted only essential oil by the headspace method. However, in this study, it is presumed that the volatile components were volatilized during the extraction process when the oil was extracted by the high-temperature compression method. This is because the main component of vegetable oil is fatty acid combined with glycerol, and the main component of essential oil is terpene. Although the proportion of volatile compounds in sancho oil is small, further research is required on compositional changes.
The main component of volatile compounds in sancho oil was estragole, which contained 95% - 96%. Bae et al. (2011) also reported that the more mature Z. schinifolium seeds, the more estragole was found among the oil components. Estragole (1-allyl-4-methoxybenzene, molecular formula: C10H12O) is a volatile phenylpropanoid belonging to a group of alkenylbenzenes such as eugenol, isoeugenol, methyleugenol, safrole, isosafrole, anethole, elemicin, myristicin, apiole (EFSA, 2009). Estragole is a major or minor component of many plants or plant parts used for herbal medicinal products, botanicals and flavourings (EMEA, 2005).
Estragole is a plant in the family Hepataceae and has been studied extensively in the essential oil of fennel, which is used as medicine, food, and spice. The essential oil consists mainly of anethol (80.0%) (a substance that is believed to be anticancer), containing less than 10% estragol and less than 7.5% fencon (Brand, 1993). Estragol is contained in basil oil extracted by steam distillation and is the main component of tarragon essential oil (60.0% - 75.0% included), pine oil, turpentine, fennel, and anise (2%). It is also used in perfume (Ashurst, 1999).
Estragole from sancho contains 0.1% - 35.0% of essential oils extracted by the headspace SPME (Solid-Phase Microextration) method (Cho et al., 2002). However, estragole has been analyzed in sancho oil. On the other hand, the main component of essential oil extracted from Z. schinifolium seeds was found to be estragole (42.0%), which was said to have a sweet herbal scent of anise-fennel (Oh and Chung, 2014).
4. Pysicochemical property of sancho oil by collection site
The chromaticity of sancho oil was investigated (Fig. 2 and Table 4). The brightness value (L) was 27.19, 27.21, 29.70, and 26.54 in Hadong, Gapyeong, Jinan, and Yeongwol sancho oil, respectively, which was the highest in Jinan, Gapyeong, Hadong, and Yeongwol, respectively.

Sancho oil extracted from seeds collected in 4 regions.Site; Hadong (H), Gapyung (G), Jinan (J) and Youngwol (Y).
All of the sancho oil in the Hadong collection sites showed negative values, which means no red or green color. The channel (b) showing the color information of blue and yellow was highest in Gapyeong, Yeongwol, Hadong, and Jinan, and the values were 38.26, 32.85, 4.32, and -0.5, respectively. Jinan Sancho oil was found to have achromatic color, Hadong contained a very light yellow color, and Gapyeong and Yeongwol sancho oil were yellow.
As a result of the chromaticity analysis, it was possible to divide into the group of sancho oil produced in Hadong and Jinan and the sancho oil produced in Gapyeong and Yeongwol. It is known that vegetable oils and fats have different colors. It is known that the color of vegetable oils and fats also shows very other aspects depending on the species.
The chromaticity of soybean oil was 0.02 (Lee et al., 2009), and in bamboo-induced colorimetric analysis, the contrast was 53.8 in the unpurified sample, and the redness was 29.1 (Na et al., 2008). It is presumed that the color difference of sancho oil extracted from the four collection sites may have been due to heating during the extraction process or low molecular weight substances contained in the sancho oil. As for the color of soybean oil, the longer the heating time, the darker the color of the oil.
The acid value of sancho oil produced by collection site was investigated (Fig. 3). The acid value of sancho oil was highest in the order of Hadong, Jinan, Gapyeong, and Yeongwol, and there was a significant difference between Hadong and Jinan, and Gapyeong and Yeongwol.

Acid value of sancho oil collected from Hadong (H), Gapyung (G), Jinan (J) and Youngwol (Y).The acid value of sancho oil and blending oil was measured according to American Oil Chemists` Society (AOCS, 1990).
The acid values of sancho oil in each collection site were found in the order of Hadong, Gapyeong, Jinan, and Yeongwol, with acid values of 1.05, 10.54, 2.63, and 5.27 ㎎/g, respectively. The acid value of sancho oil produced in Hadong and Jinan was low, and the acid value of sancho oil produced in Gapyeong and Yeongwol was very high.
The acid value indicates the degree of decomposition of fatty acids during fat and oil, that is, the degree of rancidity, and by measuring this, it is a standard for measuring the secondary oxidation of fats and oils (Yoon and Kim, 1988). Acid values vary from vegetable oil to oil and may vary depending on extracting and storage conditions. The acid value of soybean oil before heating was 0.06 ㎎/g, and the acid value of oil bone was 2.1 ㎎/g (Song et al., 2003; Lee et al., 2009).
In this study, the acid value was higher than that of other oils. In particular, sancho oil produced in Gapyeong and Yeongwol had high acidity. It is presumed that this phenomenon occurs when heat is applied during extraction and storage or the storage period is prolonged. In addition, the fact that the acid value of sancho oil is higher than that of other oils seems to be a unique characteristic. However, a detailed study on this is desired.
The viscosity of sancho oil also differed depending on the collection site (Fig. 4). The viscosity of sancho oil by collection site was in the order of Hadong, Gapyeong, Jinan, and Yeongwol, and showed values of 75.8, 76.2, 75.9, and 76.1 cP, respectively. Similar to the results of chromaticity and acid value, the viscosity could be divided into two groups: Hadong and Jinan and Gapyeong and Yeongwol (Fig. 4).

Viscocity of sancho oil collected from Hadong (H), Gapyung (G), Jinan (J) and Youngwol (Y).No statistical significance (p > 0.05).
This is a high considering that the viscosity of soybean oil, which is readily available in the market, is about 53 cps. Viscosity is generally known to increase with increasing temperature, leading to the formation of polymers in fatty acids (Al-Harbi and Al-Kabtani, 1993; Tyagi and Vasishtha, 1996).
The peroxidation value of sancho oil by collection site was 15.55 meq/㎏, 21.29 meq/㎏, 16.04 meq/㎏, and 18.51 meq/㎏ in the order of Hadong, Gapyeong, Jinan, and Yeongwol, and it was divided into Hadong, Jinan, Gapyeong and Yeongwol groups. (Fig. 5).

Peroxydation value of sancho oil collected from Hadong (H), Gapyung (G), Jinan (J) and Youngwol (Y).The peroxydation value of sancho oil was measured by the method of AOAC (1984). Means were compared at 5% significance level using Duncan's Multiple Range Test (DMRT) comparison (p < 0.05).
The peroxide value of this study was in the range of 60 meq/㎏ - 100 meq/㎏ up to 60 days of storage (Kim et al., 2010), and the peroxide value of green tea seed oil was 62 meq/㎏ - 466 meq/㎏ peroxide value (Song et al., 2003). There is a slight difference from what was shown.
In general, when vegetable oils and fats are stored for a long period, the oils and fats go rancid and have various odors. When these unsaturated fatty acids go rancid, peroxides are generated, and oils and fats stored in the air for a long time are nutritionally degraded, but the severe ones are toxic due to various peroxides. Therefore, it is very important to measure the peroxide value in oil and fat storage (Kim et al., 2010).
The fatty acid and properties of sancho oil distributed in Korea were different. This seems to be due to the different environments of wild plants. Lim et al. (2007) investigated the content variations of fatty acids and unsaturated fatty acids in 11 types of soybeans cultivated and marketed nationwide. It was reported that the content of fatty acids and unsaturated fatty acids in soybeans showed differences in content by collection site and species.
In general, the fatty acid composition of soybeans is relatively stable and is not significantly affected by environmental conditions such as weather (Wolf et al., 1982; Ohtake et al., 2001). However, it is known that unsaturated fatty acids are greatly affected by the cultivation environment (Lim et al., 2007). In polyunsaturated fatty acids, the content of linoleic acid and linolenic acid increases when grown in a low-temperature area, and the content of oleic acid, a simple unsaturated fatty acid, increases when cultivated in a high-temperature area (Howell and Collins, 1957; Wolf et al., 1982; Cherry et al., 1985). The causes include the vitality of oleoyl and linoleolyl desaturases (Cheesebrough, 1989), an increase in the solubility of O2 in the cytoplasm (Wolf et al., 1982), and it has been reported that koji plays an important role in the synthesis of fatty acids (Britz and Cavins, 1993).
Fats and oils undergo and chemical changes during transport, storage or processing. Various types of oxidation products formed in these changes adversely affect the growth of the human body when ingested. In particular, prolonged oxidized oil damages DNA or causes cancer and is related to cell aging (Shamberger et al., 1974). Therefore, quality control of sancho oil that has been used in the private sector without any safety assurance is significant nutritionally and hygienically.
The fatty acid content of sancho oil varies by collection site, and there were also differences in properties such as acid value and antioxidant activity. The content and composition of these fatty acids may vary depending on the cultivation method and variety. In addition, it is judged that there is a difference in the ingredients depending on the compression method applying heat and the extraction method without applying heat. In this study, the composition were analyzed using the compression method applied with heat, so the difference in components according to the extraction method should be studied later. However, characteristics such as the acid value of sancho oil may change during the distribution process.
Currently, there is no standardization of the production and distribution of sancho oil. In order to standardize the production of sancho oil, it is necessary to know in advance the physicochemical characteristics of the oil extracted from each region, the type and content of fatty acids and volatile compounds contained in the oil. This study is a basic study for standardization of Sancho oil production.
As a result of this study, the characteristics of sancho oil by collection site were not significantly different, but there were some differences. Therefore, standardization of the production and distribution of sancho oil produced in Korea is required. From another point of view, studies on the cultivation of high-producing varieties of sancho oil have not been conducted. Therefore, research on the development of high-yielding wild plant oil and high-yielding varieties seems to be necessary. This study is expected to serve as primary data for the standardization of production and distribution of sancho oil and the development of varieties.
Acknowledgments
This study was carried out with the support of “Forest Convergence Specialist Training Project (Project No. 2020186A00-2022-AA02)”.
References
-
Al-Harbi MM and Al-Kabtani HA. (1993). Chemical and biological evaluation of discarded frying palm oil from commercial restaurants. Food Chemistry. 48:395-401.
[https://doi.org/10.1016/0308-8146(93)90324-9]

- American Oil Chemists' Society(AOCS). (1990). AOCS official and tentative methods. 10th ed. American Oil Chemists' Society. Chicago. IL, USA. p.30-63.
- Association of Official Analytical Chemists(AOAC). (1984). Official Methods of Analysis. (14th ed.). Association of Official Analytical Chemists. Washington DC, USA. p.956.
- Ashurst PR. (1999). Food Flavorings. Springer. New York. NY, USA. p.11.
-
Bae SM, Jin YM, Jeong EH, Kim MB, Shin HY, Ro CW and Lee SC. (2011). Studies on proximate composition, fatty acids and volatile compounds of Zanthoxylum schinifolium fruit according to harvesting time. Korean Journal Medicinal Crop Science. 19:1-8.
[https://doi.org/10.7783/KJMCS.2011.19.1.001]

- Brand N. (1993). Foeniculum. In Keller K et al. (ed.). Hagers Handbuch der Pharmazeutischen Praxis. Springer. New York. NY, USA. p.156-181,
-
Britz SJ and Cavins JF. (1993). Spectral quality during pod development modulates soybean seed fatty acid desaturation. Plant Cell and Environment. 16:719-725.
[https://doi.org/10.1111/j.1365-3040.1993.tb00491.x]

- Chang KM and Kim GH. (2008). Analysis of aroma components from Zanthoxylum. Food Science And Biotechnology. 17:669-674.
-
Cheesebrough TM. (1989). Changes in the enzymes for fatty acid synthesis and desaturation during acclimation of developing soybean seeds to altered growth temperature. Plant Physiology. 90:760-764.
[https://doi.org/10.1104/pp.90.2.760]

-
Cherry JH, Bishop L, Hasegawa PM and Leffler HR. (1985). Differences in the fatty acid composition of the soybean seed protein produced in northern and southern areas of the USA. Phytochemistry. 24:237-241.
[https://doi.org/10.1016/S0031-9422(00)83527-X]

- Cho MG, Chang CS and Chae YA. (2002). Variation of volatile composition in the leaf of Zanthoxylum schinifolium Siebold et Zucc. & Zanthoxylum piperitum DC. Korean Journal of Medicinal Crop Science. 10:162-166.
-
Choochote W, Chaithong U, Kamsuk K, Jitpakdi A, Tippawangkosol P, Tuetun B, Champakaew D and Pitasawat B. (2007). Repellent activity of selected essential oils against Aedes aegypti. Fitoterapia 78:359-364.
[https://doi.org/10.1016/j.fitote.2007.02.006]

- Choung MG. (2006). Variation of oil contents and fatty acid compositions in Korean soybean germplasms. Korean Journal of Crop Science. 51:139-145.
- Chung MS. (2005) Volatile compounds of Zanthoxylum piperitum A.P. DC. Food Science and Biotechnology. 14:529-532.
- EFSA Scientific Cooperation(ESCO) Working Group on Botanicals and Botanical Preparations. (2009). ESCO report: Advice on the EFSA guidance document for the safety assessment of botanicals and botanical preparations intended for use as food supplements, based on real case studies. EFSA Supporting Publications. 7:280.
- European Medicines Agency(EMA). (2005). Committee on herbal medicinal products(HMPC): Public statement on the use of herbal medicinal products containing estragole. European Medicines Agency. Amsterdam, Netherlands. https://naturalingredient.org/wp/wp-content/uploads/Public-Statement-on-the-use-of-Herbal-Medicinal-Products-containing-Estragole.pdf, (cited by 2021 Aug 1).
-
Howell RW and Collins FI. (1957). Factors affecting linolenic and linoleic acid content of soybean oil. Agronomy Journal. 49:593-597.
[https://doi.org/10.2134/agronj1957.00021962004900110007x]

- Jeong BS and Shin MK. (1990). Hyangyak Dictionary. Youngrimsa. Seoul, Korea. p.795.
- Kim CM and Yoon HK. (1975). Analytical studies on the fatty acids and amino acids composition of Pinus koraiensis seed. Korean Journal of Agricultural Science. 2:469-474.
-
Kim HG, Kang SM, Yong SH, Seol YW, Choi EJ, Park JH, Yu CY, Solomon T and Choi MS. (2020). Antimicrobial activity against food-hazardous microorganisms, dermatophytes, and pytopathogens and antioxidative activity of sancho oil. Korean Journal of Medicinal Crop Science. 28:38-46.
[https://doi.org/10.7783/KJMCS.2020.28.1.38]

-
Kim HG, Kang SM, Yong SH, Seol YW, Kim DH, Park JH, Yu CY and Choi MS. (2020). Extending the storage periods of Zanthoxylum schinifolium seed oil using sodium bicarbonate and ascorbic acid. Korean Journal of Medicinal Crop Science. 28:421-427.
[https://doi.org/10.7783/KJMCS.2020.28.6.421]

- Kim JH. (2003). Variation of the leaf monoterpenes concentration of Zanthoxylum schinifolium at Mt. Muhak. Journal of Basic Science. 18:137-149.
-
Kim MS, Park JH, Lim HJ, Kim DS, Kim HS, Lee KT, Park YB and Shin EC. (2017). Nutritional components and physicochemical properties of lipids extracted from forest resources. Journal of the Korean Society of Food Science and Nutrition. 46:529-536.
[https://doi.org/10.3746/jkfn.2017.46.4.529]

- Kim YS, Kim R, Na MS and Choi DB. (2010). Effect of extraction process on the physicochemical characteristics of seed oil of Camellia sinensis. Applied Chemistry for Engineering. 21:148-153.
-
Lee AY, Hong ST, Jang YS and Lee JH. (2014). Lipid composition of Korean rapeseed(Brassica napus L.) cultivar and antioxidant capacity of phenolic extract. Journal of the Korean Society of Food Science and Nutrition. 43:1817-1826.
[https://doi.org/10.3746/jkfn.2014.43.12.1817]

- Lee JH, Chang KM and Kim GH. (2009). Anti-inflammatory activities of Chopi(Zanthoxylum piperitum A.P. DC) essential oil: Suppression of the inducible nitric oxide synthase and cellular adhesion. Food Science and Biotechnology. 18:1371-1378.
- Lee JW. (1998). Volatile flavor components of Korean Sancho fruit and tree(Zanthoxylum schinfolium). Korean Journal of Food and Nutrition. 11:493-498.
- Lee SJ. (1996). Korean Folk Medicine. Monographs Series No. 3. Publishing Center of Seoul National University. Seoul, Korea. p.88.
- Lim SJ, Han HK and Ko JH. (2003). Effects of edible and medicinal plants intake on blood glucose, glycogen and protein levels in streptozotocin induced diabetic rats. The Korean Journal of Nutrition. 36:981-989.
- Lim SL, Lee YH, Chi HY, Lee SJ and Kim SJ. (2007). Diversity in lipid contents and fatty acid composition of soybean seeds cultivated in Korea. Korean Journal of Crop Science. 52:348-357.
- Lee SR. (1988). Heritability and correlation coefficients in edible oil crops in Korea. Korean Journal of Plant Resources. 1:1-7.
-
Maestri DM, Labuckas DO, Meriles JM, Lamarque AL, Zygadlo JA and Guzman CA. (1998). Seed composition of soybean cultivars evaluated in different environmental regions. Journal of the Science of Food and Agriculture. 77:494-498.
[https://doi.org/10.1002/(SICI)1097-0010(199808)77:4<494::AID-JSFA69>3.0.CO;2-B]

- Na MS, Choi HS, Lee MY, Ryu SR, Park SK, Choe YD, Piao YL, Choi DB and Shin DY. (2008). A Study on physicochemical properties and components of bamboo oil from Phyllostachys nigra var. henonis by refining process. Applied Chemistry for Engineering. 19:129-132.
-
Oh M and Chung MS. (2014). Effects of oils and essential oils from seeds of Zanthoxylum schinifolium against foodborne viral surrogates. Evidence-Based Complementary and Alternative Medicine. 8:135797. https://www.hindawi.com/journals/ecam/2014/135797/, (cited by 2021 Aug 1).
[https://doi.org/10.1155/2014/135797]

-
Ohtake N, Kawachi T, Sato A, Okuyama I, Fujikake H, Sueyoshi K and Ohyama T. (2001). Temporary application of nitrate to nitrogen-deficient soybean plants at the mid- to late-stages of seed development increased the accumulation of the β-subunit of β-conglycinin, a major seed storage protein. Soil Science and Plant Nutrition. 47:195-203.
[https://doi.org/10.1080/00380768.2001.10408382]

-
Park HS, Jun DY, Fang Z, Woo MH and Kim YH. (2008). Antimicrobial activity of seeds of Zanthoxylum piperitum against oral pathogen Streptococcus mutans. Journal of Life Science. 18:167-174.
[https://doi.org/10.5352/JLS.2008.18.2.167]

- Park YH, Park JW, Kim TS, Choi SA, and Chun SJ. (1984). Triglyceride compositions of peach kernel and apricot kernel oil. Applied Biological Chemistry. 27:278-284.
- Pyo YH, Kim IS and Ahn MS. (1989). Study on the oxidative stability of Korean evening primrose oil. Korean Journal of Food and Cookery Science. 5:27-34.
- Seo KL, Lee HJ and Koh KH. (1999). Antimicrobial activity of the volatile components from fruit peel of chopi(Zanthoxylum piperitum DC). Microbiology and Biotechnology Letters. 27:179-183.
- Shamberger RJ, Andreone TL and Willis CE. (1974). Antioxidants and cancer. IV. Initiating activity of malonaldehyde as a carcinogen. Journal of the National Cancer Institute. 53:1771-1773.
- Shin AJ and Kim DH. (1982). Studies on thermal oxidation of soybean oil. I. Changes in some chemical and physical properties of a soybean oil during thermal oxidation. Korean Journal of Food Science Technology. 14:257-264.
- Shin HS. (2017). History of edible oils and fats industry in Korea. Food Science and Industry. 50:65-81.
- Song J, Bang JK, Park HW, Park CB and Seong NS. (2003). Oxidative stability and fatty acid composition during storage of Chufa oil. Journal of the Korean Society of International Agriculture. 15:31-37.
-
Tyagi VK and Vasishtha AK. (1996). Changes in characteristics and composition of oils during deep fat frying. Journal of the American Oil Chemists' Society. 73:499-506.
[https://doi.org/10.1007/BF02523926]

-
Verruck S, Balthazar CF, Rocha RS, Silva R, Esmerino EA, Pimentel TC, Freitas MQ, Silva MC, da Cruz AG and Prudencio ES. (2019). Dairy foods and positive impact on the consumer's health. Advances in Food and Nutrition Research. 89:95-164.
[https://doi.org/10.1016/bs.afnr.2019.03.002]

-
Wolf RB, Cavins JF, Kleiman R and Black LT. (1982). Effect of temperature on soybean seed constituents: Oil, protein, moisture, fatty acids, amino acids, and sugars. Journal of the American Oil Chemists' Society. 59:230-232.
[https://doi.org/10.1007/BF02582182]

- Yang WY, Pyo YH and Ahn MS. (1996). Oxidative stability and sensory evaluation of camellia oil. Korean Journal of Food and Cookery Science. 12:367-371.
- Yoon SH and Kim JW. (1988). Antioxidative effects of various antioxidants on the soybean oil. Journal of the Korean Society of Food Science and Nutrition. 17:19-23.