Radical Scavenging Active Compound Analysis in Extract of Coffee Silver Skin
This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Coffee silver skin is a by-product generated during the processing of coffee, which has recently been cultivated mainly in the southern regions of Korea. Radical scavenging active compounds were analyzed as part of a study of active compounds present in coffee silver skin.
Hot water extracts and ethanol extracts were prepared at varying concentrations (10% - 95%). Simultaneously with liquid chromatography (LC) separation, 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity was measured and mass spectrometry (MS) of the identified radical scavenging active compound peak was performed. The analysis results confirmed the presence of three compounds, eicosanoyl-5-hydroxytryptamide, docosanoyl-5-hydroxytryptamide, and tetracosanoyl-5-hydroxytryptamide. In the rapid LC-MS/MS analysis using the multiple reaction monitoring mode, each active compound showed a higher content as the ethanol concentration increased. In particular, the content of each compound was found to be significantly decreased in the extract with an ethanol concentration of 60% or less.
The three identified alkanoyl-5-hydroxytryptamide compounds are reported to possess antioxidant activity and various neurological disease-related effects. Therefore, this analysis method can be applied to research or product development using coffee silver skin
Keywords:
Coffee Silver Skin, Liquid Chromatography, Mass Spectrometry, Radical Scavenging ActivityINTRODUCTION
Coffee is a processed fruit of the coffee tree grown in tropical and subtropical climate regions centered on the equator, and is one of the most popular beverages worldwide. Due to the suitability of the growing environment, most of the coffee consumed in Korea is imported from countries such as Colombia and Brazil, and is continuously increasing to 563.65 million dollars based on the amount of imports in 2021 (aT FIS, 2022). Recently, the cultivation of coffee trees using the facility cultivation method is gradually increasing in Jeju Island, Jeonnam, and Gyeongnam regions in Korea (Moon et al., 2019).
As shown in Fig. 1, coffee silver skin is a by-product in the form of a thin film that is separated during the roasting of green beans during coffee bean processing (Gottstein et al., 2021). The amount of silver skin produced through the processing of coffee beans is known to be 4 - 5% of coffee cherry (Narita and Inouye, 2014).
It is reported that the coffee silver skin contains around 60% of dietary fiber, as well as various fatty acids and minerals (Bessada et al., 2018). In addition, active compounds such as caftaric acid, chlorogenic acid and isomer, caffeic acid, and ferulic acid are reported to be contained in coffee sliver skin (Martuscelli et al., 2021).
Coffee silver skin, which is essentially generated during the processing of coffee beans, is inevitably generated in proportion to the amount of coffee consumed domestically and abroad, and various studies are needed to utilize it. Several studies have been conducted in Korea to confirm the possibility as a food additive or to confirm the characteristics as a cosmetic material (An and Hwang, 2013; Lee et al., 2018; Im, 2021).
Research on cosmetic materials was also conducted overseas (Bessada et al., 2018; Xuan et al., 2019), and the possibility of using it for manufacturing dietary fiber-related materials or eco-friendly bio-polymer was also studied. (Gottstein et al., 2021; Ghazvini et al., 2022). In addition, studies on the antioxidant activity of the polyphenol compound contained in coffee silver skin were conducted (Regazzoni et al., 2016).
Antioxidant activity is known to be highly related to various physiological activities such as anti-inflammatory or whitening activity, and research on the development of antioxidant active materials is continuously being performed (Song and Lee, 2015; Yoo et al., 2019; Im and Lee, 2020; Kim et al., 2020). In addition, as antioxidants that can be supplied externally, synthetic antioxidants such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) are used as additives in food or related products. However, research and development to find antioxidant materials from natural products such as plant extracts have been continuously conducted due to aversion to chemically synthesized compounds (Kim et al., 2018; Kim et al., 2019).
In general, plant extracts are in the form of a mixture of numerous compounds, and the process of separating and identifying active compounds requires a lot of time and effort. As a method of overcoming these problems, research utilizing mass spectrometer (MS) linked to liquid chromatograph (LC) is being utilized (Benayad et al., 2014; Bouhafsoun et al., 2018; Lee et al., 2022b). MS data obtained for each peak after LC separation can be used as a tool for basic and preliminary qualitative analysis in plant extract research where it is difficult to obtain standards for various unknown compounds in advance. In addition, MS/MS in the form of tandem MS, which has recently been increasingly used, can perform not only scan mode analysis that can estimate molecular weight, but also product ion or precursor ion scan, and multiple reaction monitoring (MRM) mode analysis. Through these various analyses, the usability of qualitative and quantitative analysis using MS/MS has increased (Na et al., 2020; Lee et al., 2022a).
In this study, the existence of antioxidant active compounds present in coffee hide extract was confirmed using a system that measures 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity simultaneously with LC separation. After measuring the radical scavenging activity, MS and MS/MS analysis of the peak of the confirmed radical scavenging active compound was performed. MRM mode analysis conditions were set based on the MS analysis results, and the usefulness of the analysis method was reviewed through comparative analysis of samples for each extraction condition.
MATERIALS AND METHODS
1. Plant materials and reagents
Coffee silver skin was used that was separated while processing beans produced from Arabica (Coffea arabica L.) species grown in Hwasun, Jeollanam-do. Coffee bean moisture was 8.7 - 10.9%, and after roasting at 200 - 210℃ for 10 minutes, the separated silver coat was recovered as shown in Fig. 1.
Ethanol as an extraction solvent, LC grade water and methanol were purchased from Duksan (Ansan, Korea). Formic acid and 1,1-diphenyl-2-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich (St Louis, MO, USA).
2. Preparation of extraction
For the hot water extract of coffee silver skin, 200 ㎖ of distilled water was mixed with 4 g of the sample, and extraction was performed at 100℃ for 60 minutes. After cooling to room temperature, filtering was performed with a filter paper (No. 2, Whatman, Maidstone, England), and the extract was used in the experiment while being refrigerated at 4℃. 10% - 95% ethanol extract was mixed with 200 ㎖ of ethanol for each concentration in 4 g of the sample, and shaking extraction was performed at a speed of 180 rpm for 72 hours. Each extract was used in the experiment while being refrigerated at 4℃ after filtering. A syringe filter (0.45 ㎛, Whatman, Maidstone, England) was used for filtration for analysis.
3. Online LC-DPPH radical scavenging activity measurement
For online LC-DPPH radical scavenging activity measurement of coffee silver skin extract samples, analysis was performed using LC-30A (Shimadzu Co., Kyoto, Japan) liquid chromatography and Kinetex C18 (2.1 ㎜ × 100 ㎜, 1.7 ㎛, Phenomenex, Torrance, CA, USA) as a basic equipment configuration.
The sample injection volume was 2 ㎕, the column oven was maintained at 40℃, and the flow rate was maintained at 0.3 ㎖/min using 0.1% formic acid (A) and methanol (B) as a mobile phase. Gradient program for radical scavenging active compound analysis: From 0.0 to 5.0 min 80% B (isocratic), from 5.0 to 25.0 min 80% - 95% B (linear), from 25.0 to 32.0 min 95% - 100% B (linear), from 32.0 to 38 min 100% B (isocratic), from 38.0 to 38.5 min 100% - 80% B (linear), from 38.5 to 45.0 min 80% B (isocratic).
An additional LC-20AD (Shimadzu Co., Kyoto, Japan) pump was installed to mix the sample liquid separated from the column and the DPPH radical solution, and after the radical solution was mixed, it was passed through a reaction tubing with a length of 1 m for the elimination reaction. DPPH solution dissolved in methanol at a concentration of 50 μM was supplied at 0.15 ㎖/min, and the detector (SPD-10Avp, Shimadzu Co., Kyoto, Japan) wavelength was set at 515 ㎚. Additional LC profile analysis for qualitative analysis of active compounds was performed by changing the detector wavelength to 300 ㎚ while the DPPH radical solution was stopped (Lee et al., 2022a).
4. LC-MS/MS analysis of major active compounds
For LC-MS/MS analysis of the active compound, LCMS-8050 triple quadrupole mass spectrometer (Shimadzu Co., Kyoto, Japan) coupled with LC-30A (Shimadzu Co., Kyoto, Japan) liquid chromatography was used, and the same mobile phase and column conditions as those for online LC-DPPH radical scavenging activity were applied.
An electro-spray ionization (ESI) device was used for ionization for mass spectrometry of the active compound. Detailed conditions for MS and MS/MS analysis were applied and analyzed as shown in Table 1, and MS scan was conducted in the range of 100 m/z - 1,200 m/z.
Based on the results of MS analysis that confirmed molecular ion and product ion, multiple reaction monitoring (MRM) conditions were set for rapid and accurate analysis of active compounds.
For the MRM mode analysis, the same column, mobile phase and flow rate of the MS analysis method were applied, and the sample injection volume was set to 1 ㎕. Gradient program for MRM mode analysis: From 0.0 to 2.0 min 90% B (isocratic), from 2.0 to 10.0 min 90% - 100% B (linear), from 10.0 to 15.0 min 100% B (isocratic), from 15.0 to 15.5 min 100% - 90% B (linear), from 15.5 to 20.0 min 90% B (isocratic).
RESULTS AND DISCUSSION
1. Online LC-DPPH radical scavenging activity
Among various methods for measuring antioxidant activity, 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity, which is most commonly used to measure antioxidant activity of plant extract samples, has the advantage of easy comparison of results by measuring color change according to reduction of radicals (Blois, 1958; Cha, 2015; Im et al., 2017).
In general, the DPPH radical scavenging ability measurement method uses spectrophotometer-based equipment, and it is difficult to confirm information about active compounds based on the experimental results of samples in which a lot of compounds are mixed, such as plant extracts. Therefore, in order to improve the search efficiency for antioxidant active compounds, various studies are being conducted to continuously measure antioxidant activity after compound separation using liquid chromatography (LC) (Hong et al., 2014; Zhang et al., 2015; Im et al., 2017).
In the online LC-DPPH radical scavenging activity measurement system, a radical scavenging reaction occurs only during the time when the antioxidant active compound is separated, since the radical solution is constantly supplied in the middle of the connection to the detector after column separation. DPPH radical shows a purple color, but when it reacts with a compound having antioxidant activity, it becomes discolored and absorbance decreases. Therefore, reading can be facilitated by setting the polarity of the detector to the opposite (negative mode) and converting it into a chromatogram of the same form as the general LC analysis result. For detailed conditions related to this, the contents reviewed in previous studies were applied (Im et al., 2017; Kim et al., 2019; Im and Lee, 2020; Lee et al., 2022a).
As a result of measuring the online LC-DPPH radical scavenging activity of the coffee silver skin ethanol extract, three major radical scavenging active peaks were confirmed as shown in Fig. 2A. The formation of a peak in the results of the measurement system used in this study means that a compound with antioxidant activity was separated at the retention time. In addition, the larger the area of the peak indicates that the antioxidant activity may be relatively strong or the content may be high.
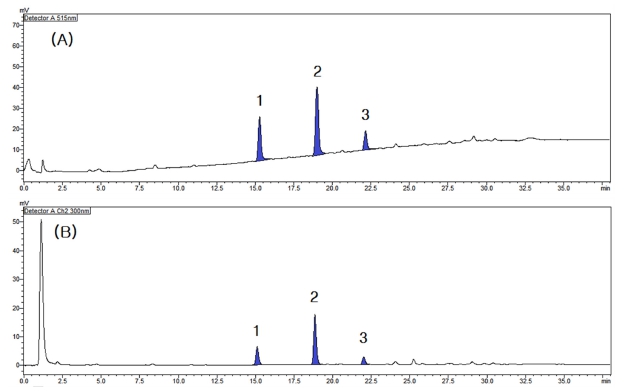
Online LC-DPPH radical scavenging effects and major active peaks of the extract of coffee silver skin.(A) DPPH radical scavenging activity profile, (B) LC profile chromatogram at 300 ㎚. Different superscripts (1 - 3) are major active peaks.
As a result of analyzing the LC profile measured at a wavelength of 300 ㎚ by applying the same LC separation conditions while the supply of the DPPH radical solution was stopped, it was confirmed that each active peak appeared in the same pattern as shown in Fig. 2B.
2. LC-MS analysis of major compound peaks
The mass spectrometry (MS) results of each active peak were reviewed based on the retention time of the peak identified in the online DPPH-radical scavenging activity measurement result and the LC profile analysis result.
The results shown in Fig. 3 were obtained by MS analysis of 95% ethanol extract of coffee silver skin. Through LC profile analysis and comparison of active peak retention time of DPPH radical scavenging ability measurement results, the molecular weight of active peaks 1 - 3 was confirmed as shown in Fig. 4 - Fig. 6.
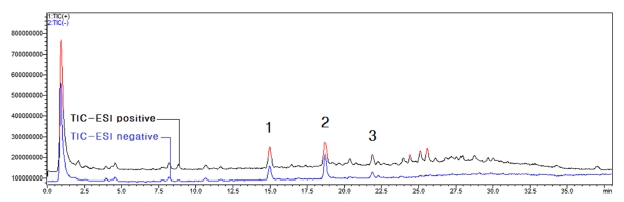
LC-MS total ion chromatograms of the extract of coffee silver skin.Different superscripts (1 - 3) are major active peaks.
In the positive mode of the MS spectra of active peak 1 shown in Fig. 4A, 471.4 m/z and 493.4 m/z, which can be seen in the form of [M+H]+ and [M+Na]+, were confirmed. In addition, as 469.4 m/z and 515.4 m/z, which can be seen in the form of [M-H]- and [M+HCOOH-H]- in the negative mode (Fig. 4B), were confirmed, it was confirmed that the compound had a molecular weight of 470.
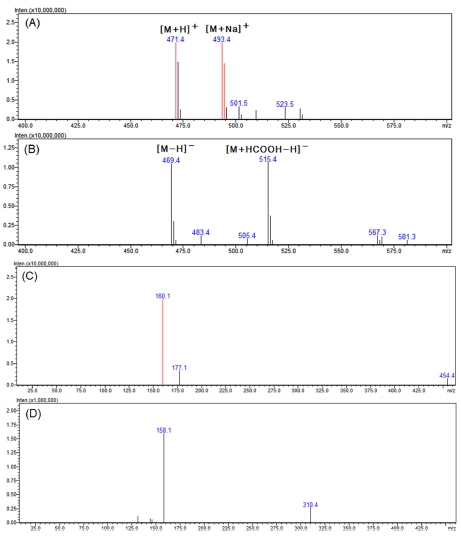
LC-MS analysis results of the major active peak 1.(A) positive mode MS spectra of the major peak 1, (B) negative mode MS spectra of the major peak 1, (C) positive mode MS2 spectra of 471.4 m/z ([M+H]+), (D) negative mode MS2 spectra of 469.4 m/z ([M-H]-).
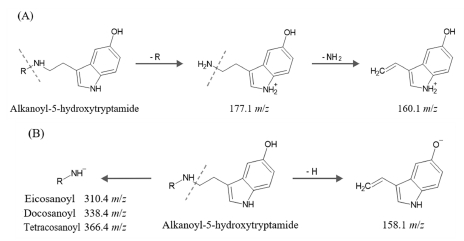
Looking at the MS2 spectra produced from 471.4 m/z in the positive mode with relatively strong molecular ions, it was found that ions of 177.1 and 160.1 m/z were characteristically generated (Fig. 4C). In the negative mode MS2 spectra, product ions of 158.1 and 310.4 m/z were generated from 469.4 m/z (Fig. 4D).
In the MS spectra for peak 2 in Fig. 5, 499.4 m/z and 521.4 m/z of the positive mode were confirmed in the form of [M+H]+ and [M+Na]+, respectively (Fig. 5A). In addition, as 497.4 m/z and 543.4 m/z of the negative mode appeared in the form of [M-H]- and [M+HCOOH-H]-, respectively (Fig. 5B), it was identified as a compound with a molecular weight of 498.
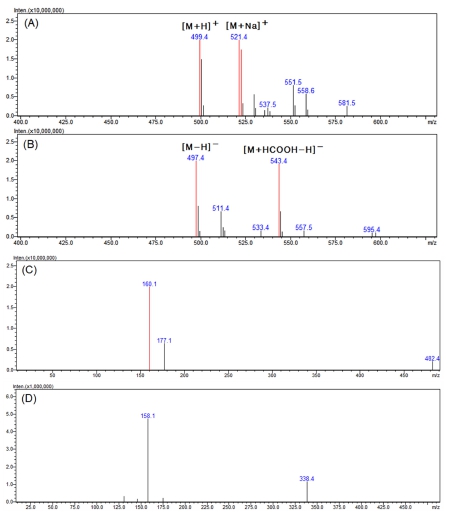
LC-MS analysis results of the major active peak 2.(A) positive mode MS spectra of the major peak 2, (B) negative mode MS spectra of the major peak 2, (C) positive mode MS2 spectra of 499.4 m/z ([M+H]+), (D) negative mode MS2 spectra of 497.4 m/z ([M-H]-).
Looking at the MS2 spectra generated from 499.4 m/z in the positive mode where the molecular ion was relatively strong, ions of 177.1 and 160.1 m/z were generated (Fig. 5C). In the negative mode MS2 spectra, product ions of 158.1 and 338.4 m/z were generated from 497.4 m/z (Fig. 5D).
In the positive mode MS spectra of active peak 3 shown in Fig. 6A, 527.5 m/z and 549.5 m/z, which can be seen in the form of [M+H]+ and [M+Na]+, were confirmed. In addition, as 525.5 m/z and 571.5 m/z in the form of [M-H]- and [M+HCOOH-H]- were confirmed in the negative mode (Fig. 6B), it was found that the compound had a molecular weight of 526.

LC-MS analysis results of the major active peak 3.(A) positive mode MS spectra of the major peak 3, (B) negative mode MS spectra of the major peak 3, (C) positive mode MS2 spectra of 527.5 m/z ([M+H]+), (D) negative mode MS2 spectra of 525.5 m/z ([M-H]-).
Looking at the MS2 spectra generated from 527.5 m/z in positive mode, ions of 177.1 and 160.1 m/z were generated (Fig. 6C). In the negative mode MS2 spectra, product ions of 158.1 and 366.4 m/z were generated from 525.5 m/z (Fig. 6D).
As shown in Fig. 7A, the 177.1 and 160.1 m/z ions commonly identified in the positive mode MS2 spectra of each peak are typical fragmentation forms in MS analysis of compounds having a 5-hydroxytryptamide structure (Lang et al., 2009; Cao et al., 2019). In the negative mode MS2 spectra, 158.1 m/z can be commonly identified as shown in Fig. 7B, and the alkanoyl-NH- fragment ion separated from the 5-hydroxytryptamide structure combined with the alkanoyl group was 310.4, 338.4, and 366.4 m/z, respectively.
By referring to the MS analysis results of each peak and the literature related to the alkanoyl-5-hydroxytryptamide compound of coffee, it was found that each peak was eicosanoyl-5-hydroxytryptamide, docosanoyl-5-hydroxyytryptamide, and tetracosanoyl-5-hydroxytryptamide (El-Hawary et al., 2022).
The three alkanoyl-5-hydroxytryptamide compounds identified as the main compounds exhibiting radical scavenging activity have been reported to show not only antioxidant activity, but also improvement of Alzheimer's disease-related cognitive and electrophysiological impairments and neuroprotection effect through several studies (Asam et al., 2017; Run et al., 2018).
Therefore, in order to develop technologies and products in related fields, additional research on extraction methods of each compound present in coffee silver skin or comparison of contents according to varieties or processing methods will be required.
3. Multiple reaction monitoring condition setting
Based on the LC-MS analysis results of the main active peaks, the molecular ions and product ions were selected, and two multiple reaction monitoring (MRM) conditions were set for each compound as shown in Table 2 to ensure qualitative and quantitative analysis method. The mobile phase conditions were set so that the analysis was completed in 20 minutes, considering the mass spectrometric characteristics of MRM mode, which can be analyzed regardless of retention time overlap or interference.
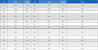
Positive ESI mode multiple reaction monitoring conditions of LC-MS/MS for analysis of radical scavenging active compounds.
In order to confirm the usefulness of the MRM mode analysis method, a comparative analysis was conducted by preparing a hot water extract and an ethanol extract by concentration. As shown in the LC-MS/MS chromatogram of MRM mode (Fig. 8), the difference in the content of each active compound was clearly confirmed for each extraction condition. By comparing the peak area of each compound for each extract condition, the relative content results are presented in Table 3.
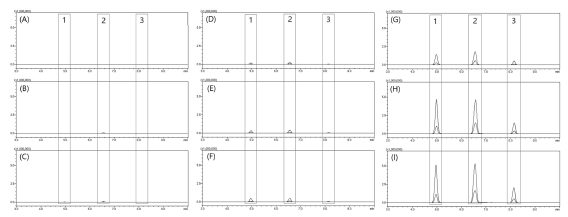
MRM mode LC-MS/MS chromatograms of active compounds by extraction solvent.(A) hot water extract, (B) 10% ethanol extract, (C) 20% ethanol extract, (D) 30% ethanol extract, (E) 40% ethanol extract, (F) 50% ethanol extract, (G) 60% ethanol extract, (H) 70% ethanol extract, (I) 95% ethanol extract. Different superscripts (1 - 3) are major active compounds.
4. Comparative analysis of radical scavenging active compounds
It is a compound with low polarity due to the structural characteristics of alkanoyl-5-hydroxytryptamide containing an alkane structure of more than 20 carbons. Therefore, it was confirmed that the extraction was easily performed in ethanol with relatively low polarity rather than in highly polar solvent conditions such as hot water. Moreover, it was confirmed that the content of each compound was significantly reduced at an ethanol concentration of 60% or less among the ethanol extracts by concentration.
Coffee trees are gradually increasing in Jeju, Jeonnam, and Gyeongnam regions in Korea using facility cultivation methods, so various studies are needed to develop related products. In order to search for antioxidant active compounds present in coffee silver skin produced during coffee processing, DPPH radical scavenging activity was measured simultaneously with LC analysis, and mass spectrometry was performed on the identified radical scavenging active compound peak.
As a result of the analysis, three active compounds were identified: eicosanoyl-5-hydroxytryptamide, docosanoyl-5-hydroxytryptamide, and tetracosanoyl-5-hydroxytryptamide. Based on the mass spectrometry results, the MRM mode LC-MS/MS rapid analysis conditions were set, and the usefulness was confirmed by conducting a comparative analysis of coffee silver skin extracts for each extraction solvent.
Through this study, three alkanoyl-5-hydroxytryptamide compounds identified as antioxidant active compounds of coffee silver skin are reported to have antioxidant activity and various neurological disease-related effects. Therefore, the results of this study are expected to be used as basic data for product development or research in related industries such as functional foods and pharmaceuticals.
References
-
An HL and Hwang YK. (2013). Quality characteristics of yellow layer cake added with coffee silver skin. The Korean Journal of Culinary Research. 19:33-45.
[https://doi.org/10.20878/cshr.2013.19.3.003]

-
Asam K, Staniszewski A, Zhang H, Melideo SL, Mazzeo A, Voronkov M, Huber KL, Pérez E, Stock M, Stock JB, Arancio O and Nicholls RE. (2017). Eicosanoyl-5-hydroxytryptamide (EHT) prevents Alzheimer's disease-related cognitive and electrophysiological impairments in mice exposed to elevated concentrations of oligomeric beta-amyloid. PLOS ONE. 12:e0189413. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0189413, (cited by 2022 Dec 18).
[https://doi.org/10.1371/journal.pone.0189413]

-
Benayad Z, Gómez-Cordovés C and Es-Safi NE. (2014). Characterization of flavonoid glycosides from fenugreek (Trigonella foenum-graecum) crude seeds by HPLC-DAD-ESI/MS analysis. International Journal of Molecular Sciences. 15:20668-20685. https://www.mdpi.com/1422-0067/15/11/20668, (cited by 2022 Dec 18).
[https://doi.org/10.3390/ijms151120668]

-
Bessada SMF, Alves RC and Oliveira MBPP. (2018). Coffee silverskin: A review on potential cosmetic applications. Cosmetics. 5:5. https://www.mdpi.com/2079-9284/5/1/5, (cited by 2023 Jan 3).
[https://doi.org/10.3390/cosmetics5010005]

-
Blois MS. (1958). Antioxidant determinations by the use of a stable free radical. Nature. 181:1199-1200.
[https://doi.org/10.1038/1811199a0]

-
Bouhafsoun A, Yilmaz MA, Boukeloua A, Temel H and Harche MK. (2018). Simultaneous quantification of phenolic acids and flavonoids in Chamaerops humilis L. using LC-ESI-MS/MS. Food Science and Technology. 38:242-247.
[https://doi.org/10.1590/fst.19917]

-
Cao Y, Wu J, Pan H and Wang L. (2019). Chemical profile and multicomponent quantitative analysis for the quality evaluation of toad venom from different origins. Molecules. 24:3595. https://www.mdpi.com/1420-3049/24/19/3595, (cited by 2019 Oct 6).
[https://doi.org/10.3390/molecules24193595]

- Cha BC. (2015). Changes in the constituents and antioxidant activity in accordance with the processing conditions of Citrus unshiu Markovich. Korean Journal of Pharmacognosy. 46:23-30.
-
El-Hawary EA, Zayed A, Laub A, Modolo LV, Wessjohann L and Farag MA. (2022). How does LC/MS compare to UV in coffee authentication and determination of antioxidant effects? Brazilian and Middle Eastern coffee as case studies. Antioxidants. 11:131. https://www.mdpi.com/2076-3921/11/1/131, (cited by 2022 Jan 7).
[https://doi.org/10.3390/antiox11010131]

-
Ghazvini AKA, Ormondroyd G, Curling S, Saccan A and Sisti L. (2022). An investigation on the possible use of coffee silverskin in PLA/PBS composites. Journal of Applied Polymer Science. 139:52264. https://onlinelibrary.wiley.com/doi/full/10.1002/app.52264, (cited by 2022 Feb 15).
[https://doi.org/10.1002/app.52264]

-
Gottstein V, Bernhardt M, Dilger E, Keller J, Breitling-Utzmann CM, Schwarz S, Kuballa T, Lachenmeier DW and Bunzel M. (2021). Coffee silver skin: Chemical characterization with special consideration of dietary fiber and heat-induced contaminants. Foods. 10:1705. https://www.mdpi.com/2304-8158/10/8/1705, (cited by 2021 July 23).
[https://doi.org/10.3390/foods10081705]

-
Hong JS, Kang BG, Jang YS, Kim SH, Wang Z, Park YH, Park JH and Lim SS. (2014). Studies on standardization of licorice based on its active components with on-line HPLC bioassay system. Korean Journal of Plant Resources. 27:401-414.
[https://doi.org/10.7732/kjpr.2014.27.5.401]

- Im DY and Lee KI. (2020). LC-MS/MS screening method for radical scavenging active compounds in extracts of Ulmus pumila cortex. Journal of Life Science. 30:956-964.
-
Im DY, Pyo BS, Kim SM and Lee KI. (2017). Measurement of the anti-oxidative properties of extract from medicinal plants using an on-line HPLC-DPPH assay. Journal of Life Science. 27:44-49.
[https://doi.org/10.5352/JLS.2017.27.1.44]

-
Im DY. (2021). Physiological and antimicrobial activities of coffee sliver skin ethanol extracts. Journal of Business Convergence. 6:9-24.
[https://doi.org/10.31152/JB.2021.08.6.3.19]

-
Kim AY, Pyo BS, Kim SM, Park MJ, Lee SS and Lee KI. (2019). Radical scavenging effects of 10 plant essential oils and active compound screening analysis. Korean Journal of Medicinal Crop Science. 27:427-435.
[https://doi.org/10.7783/KJMCS.2019.27.6.427]

-
Kim HG, Kang SM, Park DJ, Yong SH, Yang WH, Park JH, Yu CY, Solomon T and Choi MS. (2018). Effects of blending oil and antioxidants to prevent rancidity of sancho oil. Korean Journal of Medicinal Crop Science. 26:455-463.
[https://doi.org/10.7783/KJMCS.2018.26.6.455]

-
Kim SM, Kim AY and Lee KI. (2020). Nitric oxide production inhibitory effects of three caffeoylquinic acids isolated from hot water extract of Eriobotrya japonica L. leaves. Korean Journal of Medicinal Crop Science. 28:245-253.
[https://doi.org/10.7783/KJMCS.2020.28.4.245]

- Korea Agro-Fisheries and Food Trade Corporation Food Information Statistics System(aT FIS). (2022). Food market news letter. Korea Agro-Fisheries and Food Trade Corporation. Naju, Korea. https://www.atfis.or.kr, (cited by 2022 May 19).
-
Lang R, Bardelmeier I, Weiss C, Rubach M, Somoza V and Hofmann T. (2009). Quantitation of βN-alkanoyl-5-hydroxytryptamides in coffee by means of LC-MS/MS-SIDA and assessment of their gastric acid secretion potential using the HGT-1 cell assay. Journal of Agricultural and Food Chemistry. 58:1593-1602.
[https://doi.org/10.1021/jf903612h]

- Lee KE, Son SH and Kang SG. (2018). The coffee sliver skin extracts from coffee beans exhibited cosmetic properties with antioxiant activity and inhibitory effects for elastase, collagenase and tyrosinase. Journal of the Society of Cosmetic Scientists of Korea. 44:39-48.
-
Lee KI, Back JH, Pyo BS and Cha SW. (2022b). Comparison of major compounds of coffee tree leaf extract by varieties cultivated in Korea. Korean Journal of Medicinal Crop Science. 30:401-410.
[https://doi.org/10.7783/KJMCS.2022.30.6.401]

-
Lee KI, Pyo BS, Choi CH and Cha SW. (2022a). Radical scavenging active compound screening analysis in extract of Coffea arabica L. leaves. Korean Journal of Medicinal Crop Science. 30:264-277.
[https://doi.org/10.7783/KJMCS.2022.30.4.264]

-
Martuscelli M, Esposito L, Di Mattia CD, Ricci A and Mastrocola D. (2021). Characterization of coffee silver skin as potential food-safe ingredient. Foods. 10:1367. https://www.mdpi.com/2304-8158/10/6/1367, (cited by 2022 June 13).
[https://doi.org/10.3390/foods10061367]

-
Moon SY, Baek SY and Kim MR. (2019). Determination of aroma profiles of coffee cultivated in Goheung, Korea by gas chromatography–ion mobility spectrometry. Korean Journal of Food Preservation. 26:576-585.
[https://doi.org/10.11002/kjfp.2019.26.5.576]

-
Na ES, Kim SS, Hong SS, Kim KJ, Lee YJ, Lee BC and Lee SK. (2020). Development of multi-residue analytical method for 261 pesticides in herbal medicines using GC-MS/MS and LC-MS/MS. The Korean Journal of Environmental Agriculture. 39:142-169.
[https://doi.org/10.5338/KJEA.2020.39.2.19]

-
Narita Y and Inouye K. (2014). Review on utilization and composition of coffee silverskin. Food Research International. 61:16-22.
[https://doi.org/10.1016/j.foodres.2014.01.023]

-
Regazzoni L, Saligari F, Marinello C, Rossoni G, Aldini G, Carini M and Orioli M. (2016). Coffee silver skin as a source of polyphenols: High resolution mass spectrometric profiling of components and antioxidant activity. Journal of Functional Foods. 20:472-485.
[https://doi.org/10.1016/j.jff.2015.11.027]

-
Run Y, Zhang J, Park HJ, Park ES, Oh S, Zheng H, Junn E, Voronkov M, Stock JB and Mouradian MM. (2018). Synergistic neuroprotection by coffee components eicosanoyl-5-hydroxytryptamide and caffeine in models of Parkinson's disease and DLB. Proceedings of the National Academy of Sciences of the United States of America. 115:E12053-E12062. https://www.pnas.org/doi/full/10.1073/pnas.1813365115, (cited by 2022 Dec 3).
[https://doi.org/10.1073/pnas.1813365115]

-
Song JH and Lee SR. (2015). Anti-oxidant and inhibitory activity on NO production of extract and its fractions from Rosa davurica Pall. leaves. Korean Journal of Medicinal Crop Science. 23:20-26.
[https://doi.org/10.7783/KJMCS.2015.23.1.20]

-
Xuan SH, Lee KS, Jeong HJ, Park YM, Ha JH and Park SN. (2019). Cosmeceutical activities of ethanol extract and its ethyl acetate fraction from coffee silverskin. Biomaterials Research. 23:64-74.
[https://doi.org/10.1186/s40824-018-0151-9]

-
Yoo NH, Kim HK, Lee CO, Park JH and Kim MJ. (2019). Comparison of anti-oxidant and anti-inflammatory activities of methanolic extracts obtained from different parts of Cononeaster wilsonii Nakai. Korean Journal of Medicinal Crop Science. 27:194-201.
[https://doi.org/10.7783/KJMCS.2019.27.3.194]

-
Zhang H, Xi W, Yang Y, Zhou X, Liu X, Yin S, Zhang J and Zhou Z. (2015). An on-line HPLC-FRSD system for rapid evaluation of the total antioxidant capacity of Citrus fruits. Food Chemistry. 172:622-629.
[https://doi.org/10.1016/j.foodchem.2014.09.121]