
In vivo Immunomodulatory Activity of Hippophae rhamnoides Leaf and Fruit Mixture
This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Hippophae rhamnoides is a medicinal plant and potential functional food component. To date, leaf and fruit mixtures have not been studied for immune-enhancing effects. We herein examined these mixtures for such effects.
An H. rhamnoides leaf and fruit mixture (HLF) was orally administered for 4 weeks to experimental male mice in normal and cyclophosphamide-induced immunosuppressed states, and biomarkers indicating immunomodulation were measured. In immunosuppressed animals, 200 ㎎/㎏ HLF significantly increased the serum tumor necrosis factor-α levels. B cell proliferation in the spleen upon activation by lipopolysaccharide treatment was also examined and found to be significantly lower in the immunosuppressed group than in the normal control group. Administration of HLF (100 and 200 ㎎/㎏) significantly increased cell proliferation. T cell proliferation in the spleen upon activation with concanavalin A was significantly increased by HLF in both normal and immunosuppressed animals. HLF also decreased the production and secretion of the pro-inflammatory cytokine interleukin (IL)-2, and increased production of the anti-inflammatory cytokine IL-6 in splenocytes.
HLF administration enhances immune function in normal mice and regulates the immune system in immunocompromised mice.
Keywords:
Hippophae rhamnoides Leaf and Fruit Mixture, Immunomodulatory, Immunosuppressive Model, B Cell, T Cell, Interleukin 2, Interleukin 6.INTRODUCTION
Interest in immune enhancement has been increasing owing to population aging and the novel coronavirus disease (COVID-19). Research on the development of immune-enhancing materials is actively underway, especially as the consumption of functional foods for strengthening immunity increases (Hwang, 2020).
Immunity is a protective mechanism that allows the body to defend itself against antigens. It can be divided into innate and acquired immunity. The latter only exists in vertebrates and involves antigen-presenting, B, and T cells. Effector cells include B and T cells. B cells are involved in humoral immunity and secrete antibodies, whereas T cells are mainly responsible for cellular immunity and regulate immune responses by secreting various cytokines (Delves and Roitt, 2000; Hoffman et al., 2016; Park et al., 2023).
Cyclophosphamide (CPA) is used as an immunosuppressant to inhibit rejection reactions that occur during organ or bone marrow transplantation and as an anticancer drug for the treatment of malignant tumors. However, side effects such as anemia, hair loss, platelet reduction, and nephrotoxicity have been reported owing to the non-selective toxicity of CPA, which affects normal cells (Balmer and Valley 1997; Emadi et al., 2009). Therefore, immunomodulatory functional materials that can alleviate the side effects and toxicity of CPA and improve immune function in the body are sorely needed (Lee et al., 2007).
Hippophae rhamnoides (H. rhamnoides), native to the cold regions of Europe and Asia, is an ancient plant and rich nutritional ingredients that can adapt to harsh environments. Traditionally, it has been used to improve gastric function and treat cardiovascular diseases, liver damage, tendon and ligament damage, skin diseases, and ulcers (Park et al., 2018). Through various research results related to functionality and safety, H. rhamnoides has been recognized for its potential as a source of health functional foods and its possibility as a therapeutic agent (Krejcarová et al., 2015).
It exhibits various physiological activities, including antioxidant, anticancer, antihyperlipidemic, anti-obesity, anti-inflammatory, antibacterial, antiviral, neuroprotective, and liver protective activities (Ahani and Attaran, 2022; Wang et al., 2022).
H. rhamnoides contains polyphenols, flavonoids, flavonoid glycosides, carotenoids, β-sitosterol, and ursolic acid (Lee et al., 2011; Maheshwari et al., 2011; Ahmed Wani et al., 2013; Yang et al., 2013; Jeong et al., 2015), and its fruit and oil contain high levels of palmitoleic and linoleic acids (Yang and Kallo, 2001). Palmitoleic acid, a key constituent of skin fat and a principal component in cosmetic and therapeutic applications has been reported to constitute up to 43% of pulp oil. The seed oil of H. rhamnoides has been reported to contain up to 42% linoleic acid (Fatima et al., 2012).
Polyunsaturated fatty acids (PUFAs) such as linoleic acid are known to have an immunomodulatory effect (Michel et al, 2012). H. rhamnoides also contains flavonoids such as leucocyanidin, catechin, isorhamnetin, quercetin, and quassin, which enhance immunity (Thomas et al., 2003). In broiler chicks with T-2 toxin-induced immune suppression, H. rhamnoides was reported to have an immunomodulatory effect on humoral immunity when used in conjunction with glucomannan (Ramasamy et al. 2010).
Various physiological studies using H. rhamnoides have been conducted; however, the effects of H. rhamnoides leaf and fruit mixture (HLF) on immune enhancement in induced immunosuppression have not been reported. Therefore, in this study, we evaluated the immune-enhancing efficacy of HLF to verify its potential as a functional ingredient.
Extensive research reported the crucial role of antioxidant effects in maintaining the normal function of the immune system (Meydani et al., 1995). Based on prior studies (data not shown) indicating the highest antioxidant activity (DPPH free radical scavenging activity) in a specific ratio of leaf and fruit, we utilized a mixture prepared in this optimal ratio for our investigation. The marker compounds of mixture have been standardized to isorhamnetin 3.48 ㎎/g and palmitoleic acid 18.4 ㎎/g, respectively, through prior studies.
MATERIALS AND METHODS
1. HLF preparation
The leaves of H. rhamnoides were dried in a well-ventilated shade and pulverized. The sample powder was extracted using 70% aqueous ethanol. The extract was prepared by repeating the manufacturing process thrice under the same conditions.
The 70% aqueous ethanol extract was evaporated (EYELA N-1000, Tokyo Rikakikai Co. Ltd., Tokyo, Japan)under reduced pressure at 40℃ and completely dried using a freeze dryer. The fruits of H. rhamnoides were juiced and freeze dried (Lyoph-Pride 20, IlShinBioBase, Dongducheon, Korea).
The crude extracts were stored at 70℃ in deep freezer. The mixture used in the experiment was prepared by combining leaf and fruit extracts of H. rhamnoides in a ratio of 6 : 4.
2. Experimental animals
Specific pathogen-free male mice (5 weeks old) were purchased from Orient Bio, Inc., Ltd. (Seongnam, Korea). After one week of isolation and acclimation, healthy animals without any significant weight loss were chosen for the experiment.
The mice were allowed free access to pelleted diets (Cargill Inc., Minneapolis, MN, USA), and water and light were maintained on a 12 h cycle. The mice were housed in a controlled condition with a temperature of 23 ± 3℃, relative humidity of 50 ± 10%, ventilation frequency of 10–15 times/h, lighting time of 12 h, and an illumination of 150–300 lux.
3. Induction of immunosuppression and substance administration
After a week-long adaptation period, healthy animals were selected and divided into seven groups according to the randomization method (n = 10 per group) - G1; normal, G2; normal + 100 ㎎ of HLF/㎏ of body weight, G3; normal + 200 ㎎/㎏ HLF, G4; normal + 200 ㎎/㎏ positive control substance, red ginseng concentrate (6 years old, solid content 64%, ginsenoside Rg1+Rb1+Rg3 5.5 ㎎ /g, Hong Sam Jeong PLUS, Korean Red Ginseng Corp., Inc., Daejeon, Korea), G5; immunosuppressed + negative control, G6; immunosuppressed + 100 ㎎/㎏ HLF, and G7; immunosuppressed + 200 ㎎/㎏ HLF.
Throughout the experimental period, the mice were fed an AIN-93G diet purchased from Research Diets Inc. (New Brunswick, NJ, USA) and allowed free access to food and water.
To induce immunosuppression, 100 ㎎ /㎏ of cyclopho-sphamide (CPA, Sigma-Aldrich, St. Louis, MO, USA) was intraperitoneally injected one day before administration of the test substance in the immunosuppressed test groups (G5, G6, and G7).
One day after CPA administration, the test and positive control substances were orally administered at a specific time daily for four weeks.
4. Body weight measurement
To confirm the effect of immunosuppression, the health status of the experimental and dead animals was checked daily. The weight (FOB5K.3NS, Kern, Balingen, Germany) of mice was measured weekly during the experimental period.
5. Blood collection
The experimental animals were fasted for 16 h before sacrifice, and blood was collected from the orbitals of the mice after anesthesia with tribromoethanol diluted with tert-Amyl alcohol.
Blood was collected in a separate BD Vacutainer serum tube (Becton Dickinson, Holdrege, NE, USA), incubated at room temperature for 30 min, centrifuged at 3,000 rpm for 20 min, and stored in a deep freezer until analysis.
6. Hematology and serum cytokine measurement
Blood was collected from the retro-orbital venous plexus using microcapillary tubes treated with K2-EDTA (HSU-2900000, Marienfeld Superior, Lauda-Königshofen, Germany).
Blood collected in capillaries was mixed for 20 min using a Roller Mixer (Seo Kwang Co., Ltd., Seoul, Korea) and then analyzed for hematological parameters, including white blood cells (WBC), lymphocytes, neutrophils, eosinophils, basophils, and monocytes, using an automated hematology analyzer HEMAVET (Drew Scientific, Miami Lakes, FL, USA).
Serum levels of interleukin (IL)-4, IL-6, IL-12, and tumor necrosis factor (TNF)-α were measured using ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
7. Separation of spleen cells and measurement of their proliferative capacity
After addition of RPMI 1640 culture medium (Hyclone Laboratories, Logan, UT, USA), the removed spleen was pulverized through a 40 ㎜ stainless steel mesh (BD Falcon, Franklin Lakes, NJ, USA) to prepare a single-cell solution. RPMI 1640 medium was added to the pulverized single-cell solution, centrifuged at 4℃ and 1,200 rpm for 5 min, and red blood cells were removed with a red blood cell lysis buffer (Sigma-Aldrich, St. Louis, MO, USA) to obtain spleen cells.
The spleen cells were suspended in complete RPMI 1640 medium (10% FBS, 100 units/㎖ penicillin, and 100 ㎎/㎖ streptomycin), and the cell number was determined using a hemocytometer. The proliferation of spleen cells was determined using the CellTiter 96 AQueous ONE Solution Assay Kit (Promega, Madison, WI, USA).
Isolated spleen cells were seeded into a 96 well plate (1 × 105 cells/well) and cultured in a humidified CO2 incubator (311-TIF, Thermo Fisher Scientific Inc., Waltham, MA, USA) at 37℃ (5% CO2) for 48 h with complete RPMI 1640 medium, complete RPMI 1640 medium containing 5 ㎍/㎖ concanavalin A (Con A, Sigma-Aldrich, St. Louis, MO, USA), or 100 ㎍ /㎖ lipopolysaccharide (LPS, Sigma-Aldrich, St. Louis, MO, USA).
After 48 h of culture, 20 ㎕ of ONE solution was added to each well and the cells were further cultured for 2 h. The absorbance was then measured at 490 ㎚ using a SpectraMax M2 microplate reader (Molecular Devices, San Jose, CA, USA).
8. Spleen cell cytokine assay
Spleen cells were dispensed into a 48 well plate at 2.5 × 105 cells/well to measure the cytokines produced and secreted by spleen cells. Cells were stimulated with 0.5 ㎍/㎖ Con A or 100 ㎍/㎖ LPS.
After 48 h of culture, the cell culture medium was collected, centrifuged, and the supernatant was taken. IL-2, IL-5, IL-6, and interferon (IFN)-γ levels were measured using the corresponding ELISA assay kit (R&D Systems, Minneapolis, MN, USA) as per the manufacturer’s instructions.
9. Statistical analysis
Data are expressed as the means ± SEM. The results were analyzed by Student’s t-test and one-way analysis of variance (ANOVA) using GraphPad Prism 4.0 (GraphPad Software, San Diego, CA, USA). Statistical significance was set at 5% (p < 0.05).
RESULTS AND DISCUSSION
1. HLF effects on survival
Because it is very difficult to test the exact effects of immunomodulators in healthy humans, a mouse model of immunosuppression has been established to evaluate the effects of immunomodulators in vivo (Huang et al., 2007; Zhang et al., 2021).
CPA is a cytotoxic chemotherapeutic drug that plays an important role in the treatment of tumors, and it has been reported to suppress the immune function of mice when administered at a certain dose for a short period (Wang et al., 2011; Zhu et al., 2018). Here, to investigate the effect of HLF on the survival of immunosuppressed animals, the number of animals was measured daily, and the survival rate is shown in Fig. 1.
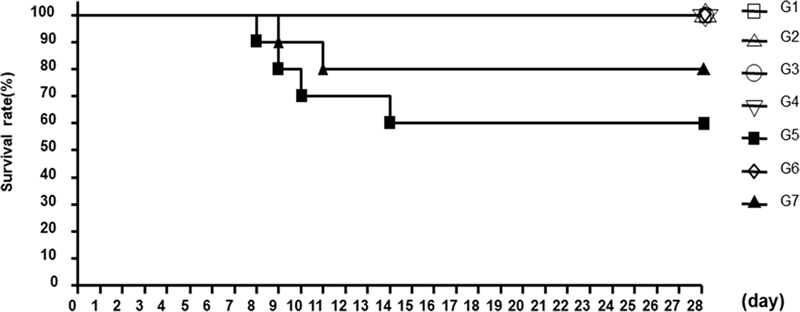
Survival rate of each group. G1; normal + control group, G2; normal + 100 ㎎/㎏ H. rhamnoides leaf and fruit mixture (HLF), G3; normal + 200 ㎎/㎏ HLF, G4; normal + 200 ㎎/㎏ positive control substance (red ginseng concentrate), G5; immunosuppressed + control group, G6; immunosuppressed + 100 ㎎/㎏ HLF, G7; immunosuppressed + 200 ㎎/㎏ HLF.
The survival rates of the test groups G1, G2, G3, and G4 were 100%, whereas the survival rate of the G5 group, which was immunosuppressed using CPA, was 60%. The survival rates of G6 and G7, which were administered 100 and 200 ㎎ /㎏ HLF, respectively, after inducing immunosuppression were 100% and 80%, respectively.
The results of this experiment in immunosuppressed mice showed that HLF intake had protective effects against immune suppression.
2. Effects of HLF on body weight
The weight of the experimental animals was measured during the experimental period, and the results are presented in Table 1.

The effect of H. rhamnoides leaf and fruit mixture (HLF) on weight gain and dietary intake in normal and immunosuppressed mice.
The weight in the normal control group (G1) increased sequentially over time, indicating a normal change in weight. The immunosuppressed control group (G5) showed significant weight loss compared with G1 in the first week.
After the first week, some animals died because of severe weight loss caused by immunosuppression. In contrast, there was no significant difference in weight in the second and third weeks compared to that in G1. However, at week 4, the weight in G5 was significantly lower than that in G1. In the normal state, HLF administration (G2 and G3) resulted in significant weight loss compared to G1 (Table 1). The daily weight gain in G1 was 0.13 ± 0.01 g, while that in G2 and G3 was significantly lower (0.06 ± 0.02 and 0.06 ± 0.01 g, respectively).
This result can be explained by the study of Jeong et al. (2015), which suggests that H. rhamnoides leaf extract inhibits the accumulation of fat in the differentiation process of 3T3-L1 cells and can prevent adipogenesis through the activation of AMP-activated protein kinase alpha (AMPKα) and inhibition of adipogenesis transcription factors.
AMPK plays an important role in promoting the oxidation of fatty acids and reducing the accumulation of adipose tissue. Adipocytokines produced and secreted by adipocytes are involved in various signaling processes. Their levels increase in the presence of obesity, suggesting a characteristic condition of chronic inflammation. Notable adipocytokines include adiponectin, leptin, TNF-a, IL-6 and IL-12. Adiponectin and leptin stimulate the fatty acid oxidation of adipose tissue by activating AMPK.
They also serve as precursors to the inflammatory process, along with other cytokines such as IL-6, IL-12, and TNF-a, and play an important role in various immune-related diseases (Gil et al., 2008; Kubota et al., 2007; Ouchi et al., 2012)
3. Effect of HLF on cell distribution
Blood samples were collected from the experimental animals and the distribution of leukocytes, which play a crucial role in the immune system, was analyzed (Table 2).

Effect of H. rhamnoides leaf and fruit mixture (HLF) on blood cell distribution in normal and immunosuppressed mice.
The total leukocyte count in the blood decreased following HLF treatment and immunosuppression induction, but there were no statistically significant differences. The leukocyte composition in the blood of the normal control group (G1) was as follows: 70.06 ± 1.89% lymphocytes and 24.93 ± 1.35% neutrophils, with eosinophils, basophils, and monocytes present in small percentages in a normal distribution. In the immunosuppressed group (G5), the proportion of lymphocytes significantly decreased and the proportion of neutrophils significantly increased. HLF administration decreased the proportion of lymphocytes and increased the proportion of neutrophils in both the normal (G2, and G3) and immunosuppressed (G6, and G7) groups. Neutrophils are rapidly activated phagocytes that are responsible for immediate inflammatory responses and effective bacterial clearance.
Jeong et al. (2020) showed that blueberry yeast-fermented powder increases the levels of neutrophils, which are immune cells in the blood, compared with a control group treated with CPA alone.
This result suggests that blueberry yeast-fermented powder may positively strengthen the immune system by increasing the levels of immune cells in the blood. Similarly, HLF was expected to increase the proportion of neutrophils and enhance their function, which is supported by our findings.
4. Effect of HLF on serum cytokine levels
Cytokines are produced by various immune cells and play key roles in immune regulation by regulating cell activation, growth, and differentiation. They can be classified according to various criteria, and those produced and secreted by helper T (Th) cells, which are the major immune cells, can be classified into Th1 and Th2 cytokines.
Representative Th1 cytokines include IL-2, IL-12, IFN-γ, and TNF-α, which mainly act on cell-mediated immune responses. IL-4, IL-6, Il-8, and transforming growth factor (TGF)-β are representative Th2 cytokines that mainly act on humoral immune responses mediated by B cells. In this study, the serum levels of the Th1 cytokines IL-12 and TNF-α and the Th2 cytokines IL-4 and IL-6 were measured to investigate the effects of HLF on the production and secretion of cytokines. Serum IL-12, IL-4, and IL-6 levels were not significantly altered by immunosuppression or HLF administration.
On the other hand, serum TNF-α levels significantly decreased following HLF administration under normal conditions. In the case of immunosuppression, TNF-α levels were significantly increased by the administration of 200 mg/kg HLF (Table 3).

Regulation of on serum cytokine levels by H. rhamnoides leaf and fruit mixture (HLF) in normal and immunosuppressed mice.
Kim et al. (2022) investigated the immunomodulatory effects of an enzymatic hydrolysate from porcine placenta, and showed that TNF-α levels significantly decrease in response to CPA treatment when compared to the levels in the normal group. In contrast, in the experimental groups treated with porcine placental enzymatic hydrolysate, all experimental groups showed significantly higher levels than the control group, showing similar results to those of HLF in this study.
TNF-α is a representative proinflammatory cytokine that acts on macrophages to promote inflammation and as a co-stimulator for activating T cells and B cells. Based on these results, it can be concluded that TNF-α levels increase when HLF is administered in a state of immunosuppression, thereby regulating the immune system.
5. Effect of HLF on splenocyte proliferation
Splenocytes were isolated from the spleens of experimental animals in each test group, and their proliferation rate was investigated. Normal proliferation rates were observed, with no significant differences between the test groups (data not shown).
To investigate the effect of HLF on B cell proliferation in the spleen, B cell activity was induced by LPS treatment and the degree of proliferation in each test group was investigated. The number of live cells in the control group without LPS treatment was 0.978 ± 0.008.
In the case of LPS treatment, the number of live cells (1.198 ± 0.007) significantly increased compared to that in the control group, indicating that B cells were strongly activated by LPS. Under normal conditions, B cell proliferation was decreased by 100 ㎎/㎏ HLF, but B cell proliferation was effectively increased by 200 ㎎/㎏ HLF. Cell proliferation in the G5 group, which had suppressed immunity, was significantly inhibited compared to that in the normal control group (G1), with a value of 0.987 ± 0.012. When HLF was administered (G6 and G7), however, cell proliferation was significantly increased to 1.147 ± 0.029 and 1.247 ± 0.032, respectively (Table 4). These results suggest that HLF enhances the humoral immune function of B cells.

Effect of H. rhamnoides leaf and fruit mixture (HLF) on LPS-induced B cell proliferation in normal and immuno-suppressed mice.
T cells are involved in cell-mediated immunity and are activated by Con A. To investigate the effect of HLF on the proliferation of T cells in the spleen, cells were activated by Con A and then cell proliferation was investigated. The number of live cells in the control group without Con A treatment was 0.799 ± 0.005, and the number of live cells in the Con A-treated group (G1) was significantly increased to 1.041 ± 0.004, indicating that T cells were activated by Con A. Cell proliferation in G5, which had suppressed immunity, was significantly decreased (0.0828 ± 0.004) compared to that in the normal control group (G1). The HLF-treated groups showed significantly increased T cell proliferation in both the normal and immunosuppressed states (Table 5).

Effect of H. rhamnoides leaf and fruit mixture (HLF) on Con A-induced T cell proliferation in normal and immunosuppressed mice.
This indicates that HLF may enhance the cell-mediated immune function of T cells. Lee et al. (2019) reported that Platycodon grandiflorum extract enhances immunity by improving the proliferative ability of T cells. Our results suggest that HLF enhances immune function through the proliferation of splenocytes and may prevent infection, and that it is a potential candidate for use in immune enhancement.
Furthermore, HLF may be a promising immunogenic substance that enhances humoral immune responses to various diseases.
6. Effect of HLF on cytokine production and secretion by splenocytes
To investigate the effect of HLF administration on cytokine production and secretion by LPS-treated and Con A-treated splenocytes, the culture supernatant of splenocytes was collected and the contents of IL-2, IFN-γ, IL-5, and IL-6 were measured. When splenocytes were stimulated with LPS, there was a significant increase in IL-2 production and secretion, while IFN-γ production and secretion significantly decreased. IL-5 and IL-6 production and secretion were not induced by LPS treatment (Table 6).
IL-2 production and secretion were also significantly increased when splenocytes were stimulated with Con A, while IFN-γ production and secretion were significantly decreased, and IL-5 and IL-6 production and secretion were unchanged (Table 7).
Under normal conditions, IL-6 production and secretion by LPS-induced splenocytes were significantly increased by the administration of HLF (200 ㎎/㎏). IL-2 production and secretion by Con A-treated splenocytes were significantly decreased by administration of 100 or 200 ㎎ /㎏ HLF (Tables 6 and Table 7).
In the immunosuppressed state, IL-2 production and secretion by LPS-induced splenocytes were significantly decreased in the group treated with HLF (100 ㎎/㎏). These results indicate that HLF decreases the production and secretion of the proinflammatory cytokine IL-2 and increases the production and secretion of the anti-inflammatory cytokine IL-6, thereby regulating the production and secretion of cytokines to exert immunomodulatory effects.
In immune responses, Th1 cells primarily promote inflammatory responses by producing cytokines like IL-2 and IFN-γ. In contrast, Th2 cells produce cytokines such as IL-5 and IL-6 to promote anti-inflammatory responses (Lee et al., 2018; Moghbeli et al., 2021).
In the immuno- suppression condition, a decrease in IL-2 levels was observed, suggesting a potential reduction in Th1 cell activity. This could indicate that the inflammatory response might have been suppressed.
Conversely, an increase in IL-6 levels under normal conditions could indicate an increase in Th2 cell activity, suggesting a possible enhancement of the anti-inflammatory response.
These results may suggest a shift in the Th1/Th2 balance towards Th2, or anti-inflammatory responses. This could indicate that HLF may regulate the immune response by suppressing Th1 cell activity and promoting Th2 cell activity, thereby adjusting the Th1/Th2 balance. However, this interpretation needs to be further validated through additional experiments and data analysis.
Acknowledgments
This study was supported by the Regional Specialized Industry Development Program (reference number R0005131). The leaves and fruits of Hippophae rhamnoides were provided by Samsung Herb Medicine Co., Ltd.
References
-
Ahani H and Attaran S. (2022). Therapeutic potential of Seabuckthorn(Hippophae rhamnoides L.) in medical sciences. Cellular, Molecular, and Biomedical Reports. 2:22-32.
[https://doi.org/10.55705/cmbr.2022.330326.1020]

- Ahmed Wani T, Wani SM, Shah AG and Masoodi FA. (2013). Optimizing conditions for antioxidant extraction from sea buckthorn leaf(Hippophae rhamnoides L.) as herbal tea using response surface methodology(RSM). International Food Research Journal. 20:1677-1681.
- Balmer C and Valley AW. (1997). Basic principles of cancer treatment and cancer chemotherapy. In DiPiro JT et al., (eds.). Pharmacotherapy: A Pathophysiologic approach. Third Edition, Stanford: Appleton and Lange. Los Altos, CA, USA. p.2403-2465.
-
Delves PJ and Roitt IM. (2000). The immune system. New England Journal of Medicine. 343:37-49.
[https://doi.org/10.1056/NEJM200007063430107]

-
Emadi A, Jones RJ and Brodsky RA. (2009). Cyclophosphamide and cancer: Golden anniversary. Nature Reviews Clinical Oncology. 6:638-647.
[https://doi.org/10.1038/nrclinonc.2009.146]

-
Fatima T, Snyder CL, Schroeder WR, Cram D, Datla R, Wishart D, Weselake RJ and Krishna P. (2012). Fatty acid composition of developing sea Buckthorn(Hippophae rhamnoides L.) berry and the transcriptome of the mature seed. PLOS ONE. 7:e3409 https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0034099, (cited by 2024 February 7).
[https://doi.org/10.1371/journal.pone.0034099]

-
Gil JH, Lee JA, Kim JY and Hong YM. (2008). Leptin, adiponectin, interleukin-6 and tumor necrosis factor-a in obese adolescents. Korean Journal of Pediatrics. 51:597-603.
[https://doi.org/10.3345/kjp.2008.51.6.597]

-
Hoffman W, Lakkis FG and Chalasani G. (2016). B cells, antibodies, and more. Clinical Journal of the American Society of Nephrology. 11:137-154.
[https://doi.org/10.2215/CJN.09430915]

-
Huang GC, Wu LS, Chen LG, Yang LL and Wang CC. (2007). Immunoenhancement effects of Huang Qi Liu Yi Tang in a murine model of cyclophosphamide-induced leucopenia. Journal of Ethnopharmacology. 109:229-235.
[https://doi.org/10.1016/j.jep.2006.07.023]

- Hwang KA. (2020). Functional food for immune regulation focusing on Korean native material. Food Industry and Nutrition. 25:11-18.
-
Jeong DY, Yang HJ, Jeong SJ, Shin DY, Lee HK and Park YM. (2020). Immunoregulatory activities of blueberry yeast fermented powder in immunosuppressed model. Journal of the Korean Society of Food Science and Nutrition. 49:433-443.
[https://doi.org/10.3746/jkfn.2020.49.5.433]

-
Jeong HJ, Park JH and Kim MJ. (2015). Ethanol extract of Hippophae rhamnoides L. leaves inhibits adipogenesis through AMP-activated protein kinase(AMPK) activation in 3T3-L1 preadipocytes. Korean Journal of Plant Resources. 28:582-590.
[https://doi.org/10.7732/kjpr.2015.28.5.582]

- Kim NK, Kim MJ, Yoon SM, Kwon MJ, Shin DY, Lee HY and Park YM. (2022). Immunostimulatory effects of enzymatic porcine placental hydrolyzate against cyclophosphamide-induced immunosuppressed model. Korean Journal of Food Science and Technology. 54:155-162.
-
Krejcarová J, Straková E, Suchý P, Herzig I and Karásková K. (2015). Sea buckthorn (Hippophae rhamnoides L.) as a potential source of nutraceutics and its therapeutic possibilities - A review. Acta Veterinaria Brno. 84:257-268.
[https://doi.org/10.2754/avb201584030257]

-
Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y, Kadowaki T. (2007). Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell metabolism. 6:55-68.
[https://doi.org/10.1016/j.cmet.2007.06.003]

-
Lee EB, Lee SH, Park YG, Choi JH, Lee HK, Jang HH, Hwang GA, Hoe JS, Park SY, Choi AJ, Hwang IG, Kim JS, Lee HJ, Lee SJ and Jeong HC. (2019). Platycodon grandiflorum extract ameliorates cyclophosphamide-induced immuno-suppression in mice. Journal of the East Asian Society of Dietary Life. 29:303-309.
[https://doi.org/10.17495/easdl.2019.8.29.4.303]

-
Lee JW, Park JH, Kim JS, Choi EY, Han SN, Seong ES, Yoo CY, Kwon YS and Kim MJ. (2011). Isolation of flavonol glycoside related to antioxidant activity from Hippophae rhamnoides leaves. Korean Journal of Medicinal Crop Science. 19:251-256.
[https://doi.org/10.7783/KJMCS.2011.19.4.251]

-
Lee SY, Lee H, Go EJ, Park YC, Choi SK, Yu CY and Lim JD. (2018). Effect of Astragalus membranaceus polysaccharides on improves immune response anfter exhaustive exercise rats. Korean Journal of Medicinal Crop Sciece. 26:72-81.
[https://doi.org/10.7783/KJMCS.2018.26.1.72]

- Lee YS, Lee GH, Park JH, Kwon YK and Shin SW. (2007). Water extracted Evodiae fructus possesses immunomodulatory activities on cyclophosphamide-induced immune suppression. Korean Journal of Physiology and Pathology. 21:1450-1455.
-
Maheshwari DT, Kumar MSY, Verma SK, Singh VK and Singh SN. (2011). Antioxidant and hepatoprotective activities of phenolic-rich fraction of seabuckthorn(Hippophae rhamnoides L.) leaves. Food and Chemical Toxicology. 49:2422-2428.
[https://doi.org/10.1016/j.fct.2011.06.061]

-
Meydani SN, Wu D, Santos MS and Hayek MG. (1995). Antioxidants and immune response in aged persons: overview of present evidence. The American Journal of Clinical Nutrition. 62:1462-1476.
[https://doi.org/10.1093/ajcn/62.6.1462S]

-
Michel T, Destandau E, Le Floch G, Lucchesi ME and Elfakir C. (2012). Antimicrobial, antioxidant, and phytochemical investigations of sea buckthorn(Hippophae rhamnoides L.) leaf, stem, root, and seed. Food Chemistry. 131:754-760.
[https://doi.org/10.1016/j.foodchem.2011.09.029]

-
Moghbeli M, Khedmatgozar H, Yadegari M, Avan A, Ferns GA and Ghayour Mobarhan M. (2021). Cytokines and the immune response in obesity-related disorders. Advances in Clinical Chemistry. 101:135-168.
[https://doi.org/10.1016/bs.acc.2020.06.004]

-
Ouchi N, Ohashi K, Shibata R, Murohara T. (2012). Adipocytokines and obesity-linked disorders. Nagoya Journal of Medical Science. 74:19-30.
[https://doi.org/10.1016/j.cardfail.2013.08.094]

-
Park JH, Lee CO, YOO JH, Nguyen TLA, YOO NH and Kim MJ. (2018). Antioxidative and inhibitory activities of extract and juice powder from Hippophae rhamnoides L. against nitric oxide and elastase production. Korean Journal of Medicinal Crop Science. 26:119-126.
[https://doi.org/10.7783/KJMCS.2018.26.2.119]

-
Park YM, Lee HY, Shin DY, Lee L and Kim JG. (2023). Immune-boosting effects of Chrysanthemum zawadskii Herbich var. latilobum Kitamura extract on cyclophosphamide-induced immunosuppressed Sprague-Dawley animal model. Korean Journal of Medicinal Crop Science. 31:81-91.
[https://doi.org/10.7783/KJMCS.2023.31.2.81]

-
Ramasamy T, Varshneya C, and Katoch VC. (2010). Immunoprotective effect of seabuckthorn(Hippophae rhamnoides) and glucomannan on T-2 toxin-induced immunodepression in poultry. Veterinary Medicine International. 2010:149373. https://www.hindawi.com/journals/vmi/2010/149373/, (cited by 2023 August 16).
[https://doi.org/10.4061/2010/149373]

- Thomas SCL. Thomas HB and B Dave O. (2003). Nutritional and medicinal values. In Li TSC and Beveridge THJ. (eds.). Sea buckthorn(Hippophae rhamnoides L.): Production and utilization. National Research Council of Canada, Ottawa, Canada. p.101-106.
-
Wang H, Wang M, Chen J, Tang Y, Dou J, Yu J, Xi T and Zhou C. (2011). A polysaccharide from Strongylocentrotus nudus eggs protects against myelosuppression and immunosuppression in cyclophosphamide-treatedmice . International Immunopharmacology. 11:1946-1953.
[https://doi.org/10.1016/j.intimp.2011.06.006]

-
Wang Z, Zaho F, Wei P, Chai X, Hou G and Meng Q. (2022) Phytochemistry, health benefits, and food applications of sea buckthorn(Hippophae rhamnoides L.): A comprehensive review. Frontiers in Nutrition. 9:1036295. https://www.frontiersin.org/articles/10.3389/fnut.2022.1036295/full, (cited by 2023 August 31).
[https://doi.org/10.3389/fnut.2022.1036295]

-
Yang B and Kallio HP. (2001). Fatty acid composition of lipids in seabuckthorn(Hippophae rhamnoides L.) berries of different origins. Journal of Agricultural and Food Chemistry. 49:1939-1947.
[https://doi.org/10.1021/jf001059s]

-
Yang ZG, Wen XF, Li YH, Matsuzaki K and Kitanaka S. (2013). Inhibitory effects of the constituents of Hippophae rhamnoides on 3T3-L1 cell differentiation and nitric oxide production in RAW264.7 cells. Chemical and Pharmaceutical Bulletin. 61:279-285.
[https://doi.org/10.1248/cpb.c12-00835]

-
Zhang J, Gao S, Cao M, Li H, Cao M, Li W and Liu X. (2021). Immunomodulatory effects of selenium-enriched peptides from soybean in cyclophosphamide-induced immunosuppressed mice. Food Sciences and Nutrition. 9:6322-6334.
[https://doi.org/10.1002/fsn3.2594]

-
Zhu G, Luo J, Du H, Jiang Y, Tu Y, Yao Y and Xu M. (2018). Ovotransferrin enhances intestinal immune response in cyclophosphamide-induced immunosuppressed mice. International Journal of Biological Macromolecules. 120:1-9.
[https://doi.org/10.1016/j.ijbiomac.2018.08.058]
