
Anti-diabetic Effect of Opuntia humifusa Extracts in Diabetic db/db Mice
This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
This study examined the anti-diabetic effects of Opuntia. humifusa extract (OHE) in diabetic db/dbmice.
The 2,2-diphenyl-1-picrylhydrazyl free radical scavenging activity and α-glucosidase and α-amylase inhibitory activities of OHE (oral administration for 4 weeks) was evaluated using biochemical parameters and histological analyses of the pancreas. Oral administration of OHE reduced plasma glucose and fructosamine levels, without affecting body weight or food intake. Additionally, it improved blood lipid parameters and restored the of islet structure in diabetic db/db mice.
The present study provides evidence for the anti-diabetic effect of OHE in db/db mice. These results suggest that OHE can be used as a medicinal supplement or functional foods for anti-diabetic therapy.
Keywords:
Opuntia. humifusa, Anti-diabetes, Hyperglycemia, HyperlipidemiaINTRODUCTION
Diabetes includes type 1 caused by β-cell destruction on Langerhans' island, and type 2 due to insulin secretion capacity and increased insulin resistance due to impaired insulin secretion due to genetic abnormalities (Isomaa et al., 2001; Goldstein, 2002; Lee et al, 2021).
In particular, type 2 diabetes is a chronic metabolic disease and has many limitations due to side effects and high failure rates of pharmacological treatment (Liu et al., 2001). Therefore, the development of new natural ingredients for the treatment of diabetes without side effects has received considerable attention.
Opuntia humifusa, which a member of the Cactaceae family that has been widely cultivated for a long time in Korea during winter. O. humifusa extract (OHE) contains minerals such as such as Mg2+, Ca2+, and K+ (Kang et al., 2012) and phenolic compounds such as gallic acid, chlorogenic acid, caffeic acid, ferulic acid, quercetin, and rutin (Cho et al., 2006; Yoo et al., 2020), which are widely used as a nutritional supplements. Because these ingredients have various effects such as antioxidant, anti-inflammation, protect of gastritis (Ngoc et al., 2023; Yoo et al., 2020)
For several years, the physiological and pharmacological properties of OHE have been studied. O. humifusa have revealed the radical scavenging and anti-inflammatory effects (Cho et al., 2006), anti-cancer (Chavez-Santoscoy et al., 2009), anti-photoaging (Han et al., 2018), anti-obesity (Yang et al., 2019). Also, O. humifusa shows have hypoglycemic and hypolipidemic effects in streptozotocin-induced diabetic rats (Hahm et al., 2011).
Nevertheless, there have not been any reports on the mechanism of the pronounced effects of anti-diabetics of OHE. In this study, we investigated the inhibitory effect of OHE for α-glucosidase and α-amylase inhibition and anti-oxidant activity in in vitro study, and hypoglycemic and hypolipidemic effect in db/dbmice.
MATERIALS AND METHODS
1. Preparation of Opuntia humifusa extract
The dried O. humifusa was manufactured by PCC Corporation, (Pyeongchang, Gangwon-do, Korea). The 70% ethanol extract of O. humifusa (OHE) was prepared by using 100 g of dried O. humifusa with 500 ㎖ of 70% ethanol at 70℃ for 3 h and then centrifuged and filtered with 55 ㎛ bag filter.
The extract solution was evaporated under 40 ㎜Hg using a rotary evaporator (N-1110S-W, Eyela, Tokyo, Japan) and lyophilized using freeze-dryer (-50℃, ILShin BioBase, Co., Ltd., Dongducheon, Korea), and then used for each experiment.
2. Animals and administration of OHE
7 week-old male C57BL/KsJ-db/db micc with genetic diabetes due to a leptin receptor gene modification on chromosome 4 were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and adapted to the following conditions for 7 days; 12 h light / 12 h dark cycle, temperature; 23 ± 1℃, humidity; 50 ± 5%, and illumination, 150 lux - 300 lux. The animals were allowed ad libitum access to food (Purina diet; Purina Korea, Seongnam, Korea) and water. After a week of acclimatization, diabetic db/db mice were treated with OHE (30, 100, and 300 ㎎/㎏) for 4 weeks. Oral administration was performed once daily.
Food intake and body weight were recorded every week. Blood glucose level was measured from the tail vein at -30, 0, 30, 60, 90, and 120 min following glucose administration. After respiratory anesthesia (isoflurane, 3 ㎖/min), the mice were sacrificed and blood samples were collected from abdominal vein.
The protocols used for the animal studies were approved by the Committee on Care and Use of Laboratory Animals of the INVIVO (Nonsan, Korea; approval no. IV-RB-17-1907-21).
3. α-Glucosidase assay
Yeast α-glucosidase (0.5 U; Sigma-Aldrich, Saint Louis, MO, USA) dissolved in 100 mM sodium phosphate buffer (pH 6.9) was mixed with various concentrations of OHE.
After incubation at 37℃ for 10 min, 3 mM p-nitrophenyl-α-d-glucopyranoside was added. The reaction was further incubated at 37℃ for 30 min and then stopped by adding of 0.1 M Na2CO3.
Absorbance of resulting p-nitrophenol was measured at 405 ㎚. Control contained reaction mixture, without any sample. Mixtures without enzyme, extract served as a blank. The percent inhibition of α-glucosidase was calculated as [(absorbance of sample - absorbance of blank) / absorbance of control] × 100. Measurements were performed in triplicate.
4. α-Amylase activity
Sample solution (200 ㎕) and 0.02 M sodium phosphate buffer (pH 6.9 with 6 mM sodium chloride, 500 ㎕) containing α-amylase solution (1 U/㎖; Sigma-Aldrich, Saint Louis, MO,) were incubated at 25℃ for 10 min. After pre-incubation, 500 ㎕ of a 1% starch solution in 200 mM sodium phosphate buffer was added. The reaction mixture was then incubated at 25℃ for 10 min. The reaction was stopped with 48 mM of dinitrosalicylic acid (Sigma-Aldrich, Saint Louis, MO, USA).
The reaction mixture was then diluted after adding distilled water, and absorbance was measured at 540 ㎚ with ELISA microplate reader (Tecan, Männedorf, Switzerland). Measurements were performed in triplicate.
5. Free radical scavenging activity
The scavenging activity of 2,2-diphenyl-1-picrylhydrazyl (DPPH; Sigma-Aldrich, Saint Louis, MO, USA) free radical was assayed according to the method of Gao et al. (2011).
The samples were diluted by phosphate buffered saline (PBS) and 100 ㎕ of the sample and 100 ㎕ of 300 mM DPPH were mixed and reacted at 37℃ for 30 minutes and the absorbance was measured at 515 ㎚. The antioxidant activity of the extracts according to the concentration was calculated by the following formula.
Scavenging activity (%) = [1 - (absorbance of extract group / absorbance of group without extract)] × 100
6. Biochemical analyses
This method was performed as previously described (Kang et al., 2012). Whole blood glucose levels were determined using Accu-Chek Aviva glucose monitoring system (Roche Diagnostics, Indianapolis, IN, USA) and plasma insulin and was measured by mouse insulin ELISA kit (Alpco Diagnostics, Wellesley, MA, USA).
The plasma concentration of fructosamine, triglyceride (TG), total cholesterol (TC), and low-density lipoprotein (LDL), high-density lipoprotein (HDL), very low-density liporotein (VLDL) -were measured by commercially available kits (Asan Pharmaceutical, Seoul, Korea).
7. Histological analysis
This method was performed as previously described Kang et al. (2012).
After the animals were killed, pancreas was removed, weighed, and fixed in 10% neutral buffered formalin.
The organ was then processed for embedding in paraffin, after which they were sectioned into 4 ㎛ - 7 ㎛ thick slices using a microtome (Thermo Scientific, Waltham, MA, USA).
The sectioned tissues were then stained with hematoxylin and eosin (H&E). Tissue damage was assessed under an optical microscope (Olympus, Fukuoka, Japan).
8. Statistical analysis
Results were analyzed by one-way analysis of variance and Duncan’s Multiple Range Tests (DMRT, p < 0.05) using SAS software (version 9.3, SAS Institute Inc., Cary, NC, USA).
RESULTS
1. Free radical scavenging activity of OHE
The DPPH radicals are widely used to evaluate the radical scavenging ability (Dinis et al., 1994). The soluble free radical DPPH is known as a hydrogen extractant that produces DPPH reduced by antioxidants (du Toit et al., 2001).
OHE and ascorbic acid (positive control) were measured on the scavenging activity against stable DPPH radicals in Fig. 1A. OHE show that the antioxidant effect increased dose-dependent (from 100 ㎍/㎖) in DPPH radical scavenging activity (Fig. 1A). This result indicated that OHE has antioxidant effect.
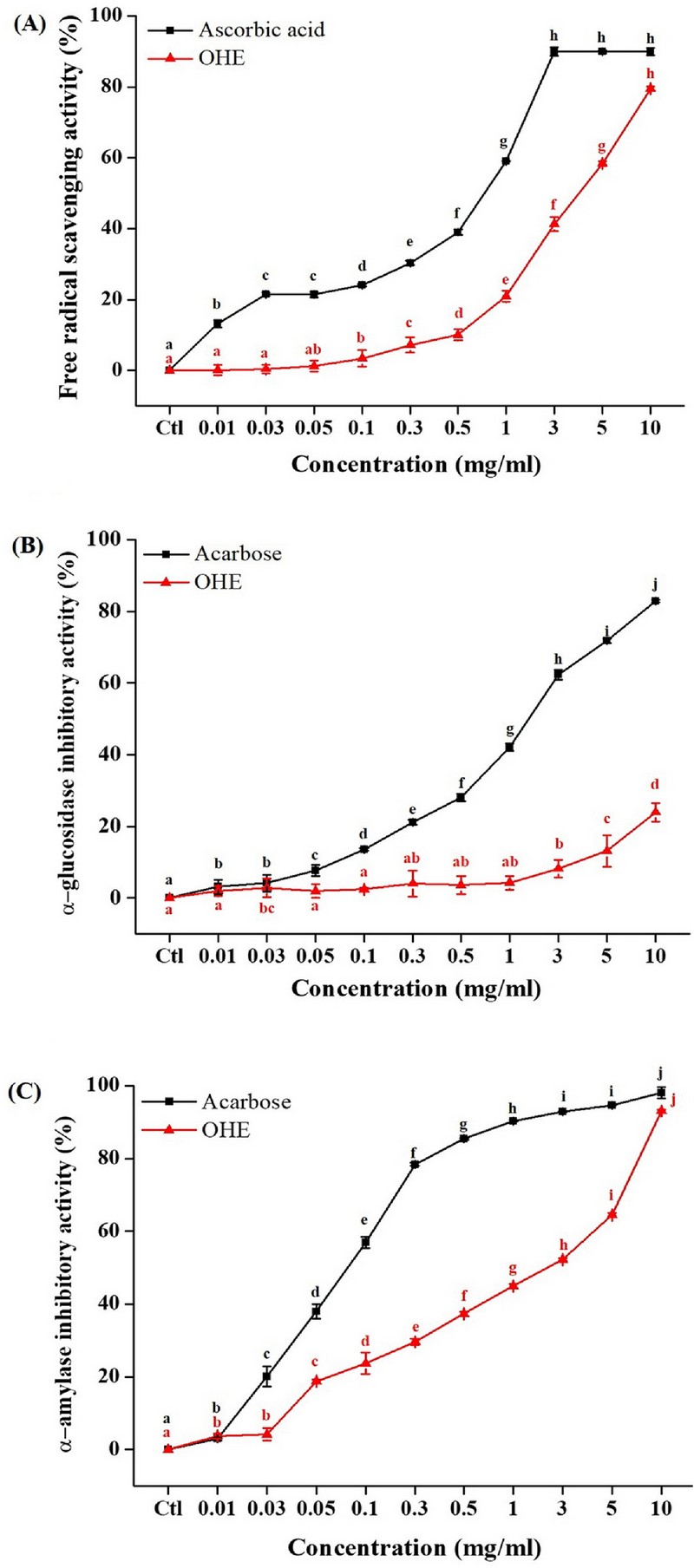
Antioxidant effect and anti-diabetic effect (α-glucosidase and α-amylase inhibitory activity) of OHE. (A) DPPH free radical scavenging activity of OHE and ascorbic acid (positive control) (B) α-glucosidase inhibitory activity and (C) α-amylase inhibitory activity of OHE and acarbose (positive control). Data are presented as means ± standard errors (n = 3). Bars labeled with different superscripts have significantly different values at 5% using Duncan’s Multiple Range Tests (DMRT, p < 0.05).
2. OHE inhibits α-glucosidase activity
The α-glucosidase is indicated on the diabetes effect, because this enzyme is involved in complex carbohydrate digestion of food-absorbing monosaccharides (Kumar et al., 2011).
Therefore, the effects of OHE was investigated on α-glucosidase activity (Fig. 1B). As shown in Fig. 1B, OHE was significantly inhibited α-glucosidase activity from 3,000 ㎍/㎖. This result show that OHE should be regarded as an effective α-glucosidase inhibitor.
3. OHE inhibits α-amylase activity.
The α-amylase, which secreted by the pancreas and salivary glands is involved in complex carbohydrate digestion of food- absorbing mono-, di-, polysaccharides (Koh et al., 2010).
Therefore, the effects of OHE was were investigated on α- amylase activity (Fig. 1C). As shown in Fig. 1B, OHE was significantly inhibited from low-concentrations (10 ㎍/㎖). This results indicated that the OHE should be regarded as an effective α-amylase inhibitor.
An oral glucose tolerance test (OGTT) was performed to confirm the effects of OHE on glucose tolerance after 4 weeks of OHE treatment (Fig. 2).
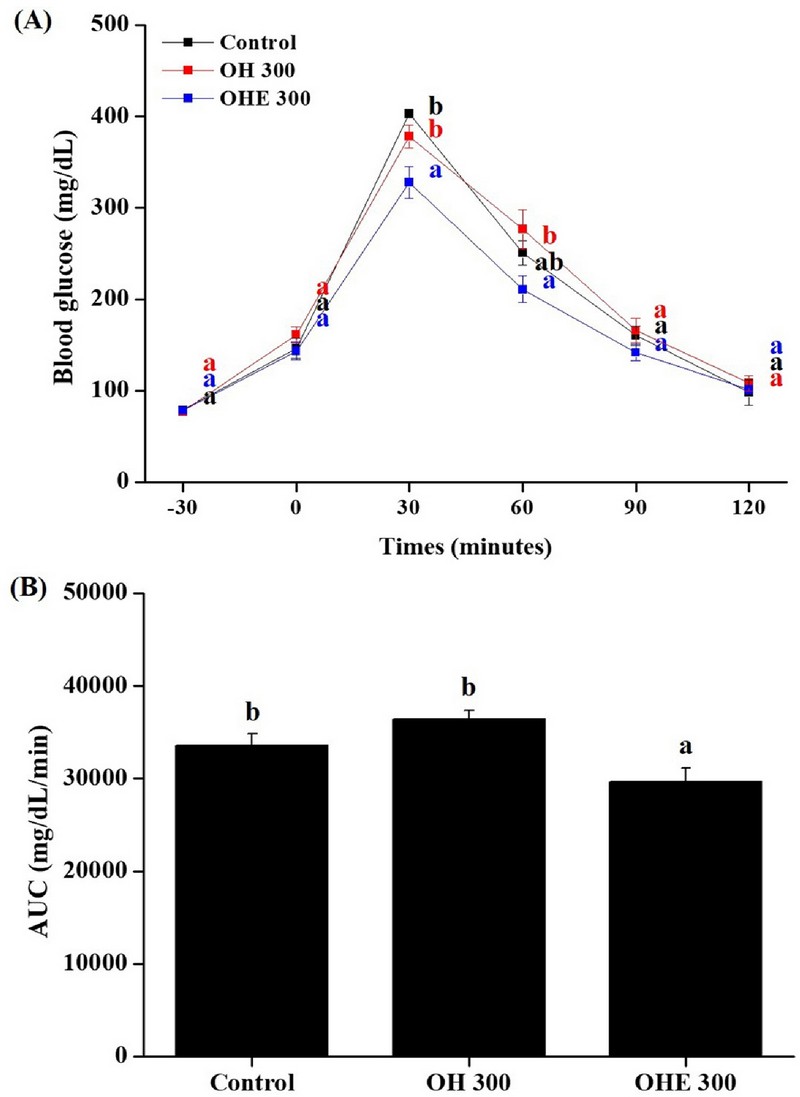
Oral glucose tolerance tests of OHE.At the end of the 4 weeks, (A) glucose concentrations and (B) area under curve (AUC) during oral glucose tolerance tests in overnight-fasted mice. Areas under the curve of blood glucose were compared. Data are presented as means ± standard errors (n = 7). Bars labeled with different superscripts have significantly different values at 5% using Duncan’s Multiple Range Tests (DMRT, p < 0.05).
The OGTT is a study that evaluates glucose resistance and insulin resistance. After fasting for 12 h, OGTT measured 2 g/㎏ of glucose by oral administration. Monitor every 30 min after glucose challenge. The area under curve (AUC) for plasma glucose is shown as a Fig. 2B.
The AUC for glucose response of OHE-treated ICR mice was significantly reduced in dose-dependent manner. This model evaluates the ability for OHE to lower blood glucose, which can reduce AUC by reducing the level of glucose migration after meals. Through these results, OHE shows that it can effectively control glucose tolerance reactions.
4. OHE ameliorates hyperglycemia and dyslipidemia in db/db mice.
To further confirm the therapeutic effect of OHE on diabetes, we investigated the anti-diabetic effect of OHE (30, 100, and 300 ㎎/㎏, experimental groups), and metformin (100 ㎎/㎏, positive group) administered for 4 weeks in db/db mice.
In the OHE and metformin intake groups, food intake and body weight were significantly increased compared to the normal group. However, it was not significantly differenced between the control group and OHE groups (Fig. 3A and B). Then, all experimental groups measured postprandial blood glucose once a week (Fig. 3C).
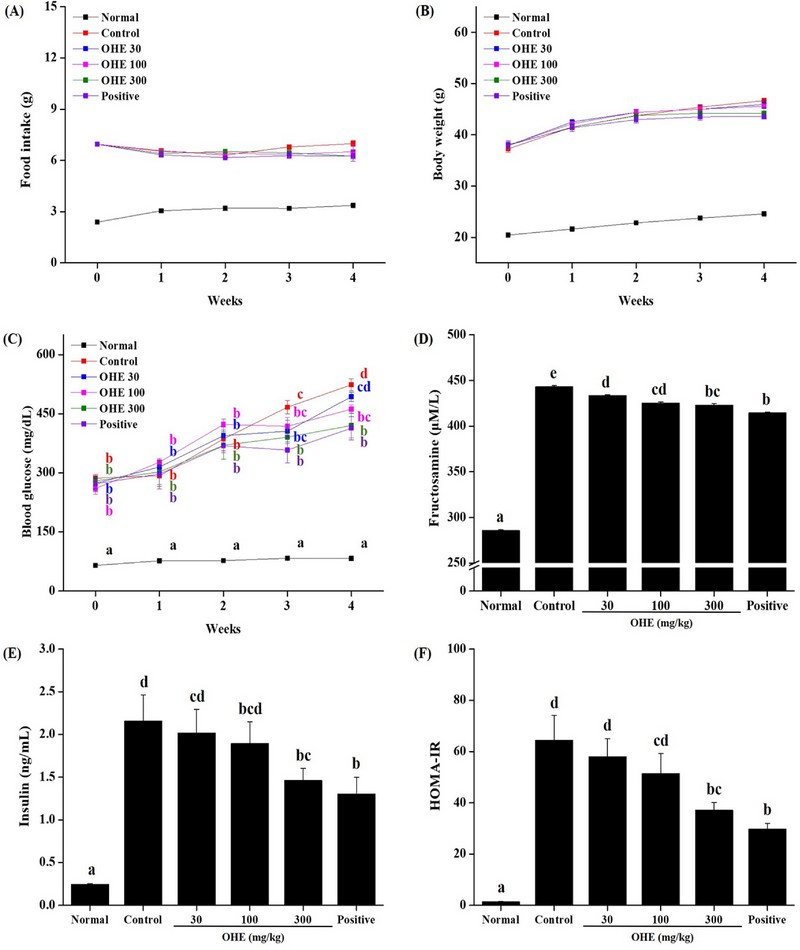
OHE improves glycemic control in db/db mice.Mice received PBS, OHE (30, 100, and 300 ㎎/㎏), and Positive (metformin, 100 ㎎/㎏) once daily for 4 weeks. (A) food intake and (B) body weight change were measured for every weeks. At the end of study, (C) fasting blood glucose levels, (D) fructosamine, (E) insulin concentrations were analyzed and (F) HOMA-IR scores were calculated. Data are presented as means ± standard errors (n = 7). Bars labeled with different superscripts have significantly different values at 5% using Duncan’s Multiple Range Tests (DMRT, p < 0.05).
Increased postprandial blood glucose was observed in all experimental groups compared to the normal group, but from 3 weeks, the postprandial blood glucose in the experimental group (OHE and positive groups) compared to the control group was significantly reduced. Additionally, levels of fructosamine, insulin and homeostatic model assessment for insulin resistance (HOMA-IR) were significantly decreased compared with the PBS-treated (normal group) db/db mice (Fig. 3D, E, and F). These results show that OHE improved the prevented antidiabetic in db/db mice.
5. Plasma lipid and cholesterol profiles in db/db mice fed OHE.
For the lipid regulating effect of OHE, we analyzed TG, TC, HDL, LDL, and VLDL in the collected plasma from normal group, control group, OHE groups and positive group. Plasma TG, TC, HDL, and LDL, and VLDL were significant increased in the control group compared to the normal group (Fig. 4).
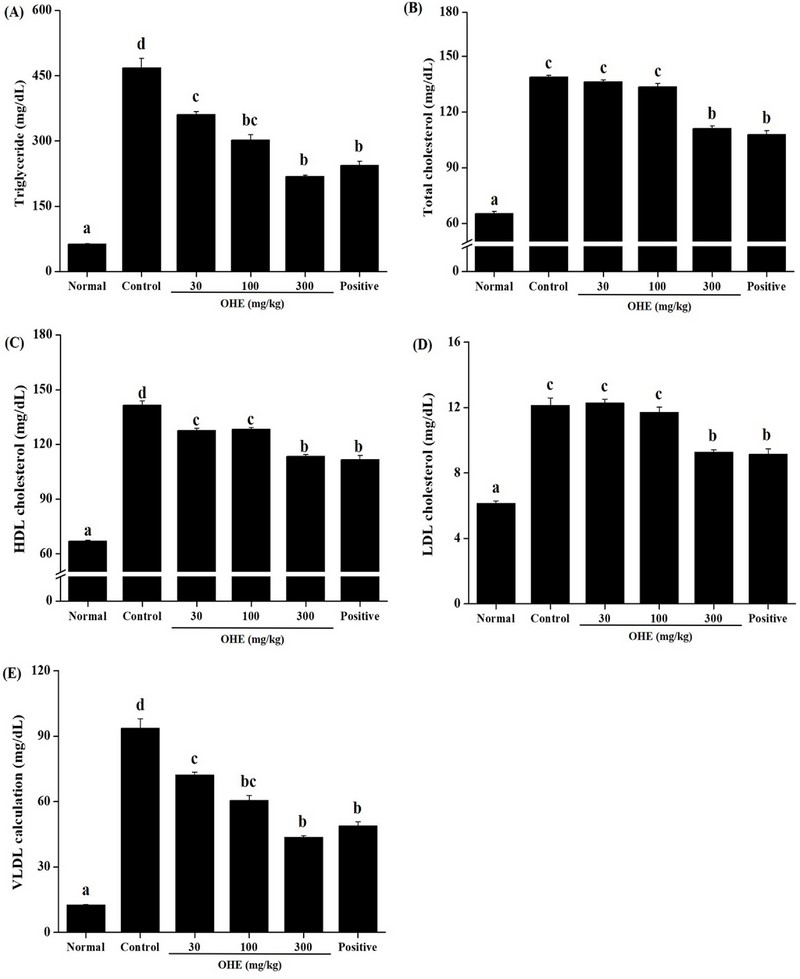
OHE improves Plasma lipid profiles in db/db mice. Mice received phosphate buffered saline (PBS), OHE (30, 100, and 300 ㎎/㎏), and positive (metformin, 100 ㎎/㎏) once daily for 4 weeks. At the end of the study, plasma levels of (A) triglyceride (TG), (B) total cholesterol (TC), (C) high desity lipoprotein-(HDL) cholesterol, (D) low density lipoprotein-(LDL) cholesterol, (E) very low-density lipoprotein (VLDL) cholesterol were determined. Data are presented as the mean ± standard error (n = 7). Bars labeled with different superscripts have significantly different values at 5% using Duncan’s Multiple Range Tests (DMRT, p < 0.05).
Level of plasma TG investigated the concentration-dependent reduction at OHE groups (30, 100, and 300 ㎎/㎏). In the plasma cholesterol, level of TC, HDL, LDL and VLDL showed a tendency to decrease compared to the control group, and showed similar results to the positive group (metformin, 100 ㎎/㎏) at high-dose of OHE (300 ㎎/㎏) (Fig. 4B, C, D, and E).
6. OHE protects pancreatic β-cells in db/db mice.
Finally, whether OHE affects the distribution of pancreatic β-cells, we stained by H&E and analyzed the pancreas of all experimental group (Fig. 5).
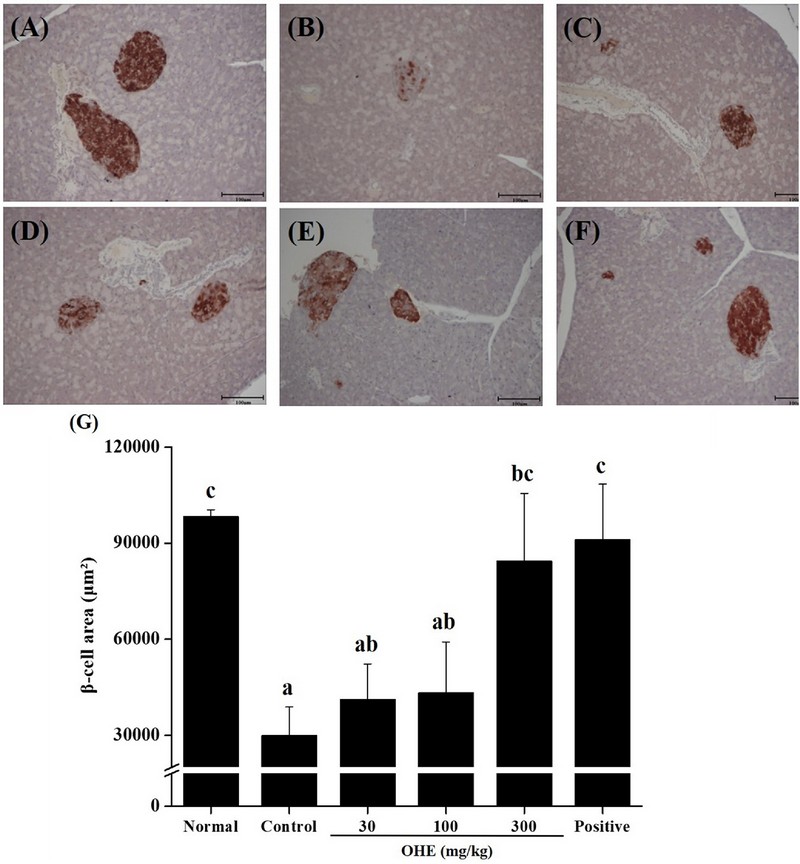
OHE prevents pancreatic islet destruction in db/db mice. Mice received phosphate buffered saline (PBS), OHE (30, 100, and 300 ㎎/㎏), and positive (metformin, 100 ㎎/㎏) once daily for 4 weeks. At the end of experiment, pancreatic tissue was isolated from all experimental mice, and pancreas sections were stained with H&E (magnification, × 10). (A) normal, (B) control, (C) OHE 30 ㎎/㎏, (D) OHE 100 ㎎/㎏, (E) OHE 300 ㎎/㎏, (F) positive (metformin, 100 ㎎/㎏), (G) the distribution of β-cells. Data are presented as the means ± standard error (n = 7). Bars labeled with different superscripts have significantly different values at 5% using Duncan’s Multiple Range Tests (DMRT, p < 0.05).
It was investigated that the distribution of β-cells, in the control group was significantly reduced compared to the normal group (Fig 5A and B). In addition, dose-dependent recovery was observed in the OHE groups (30, 100, and 300 ㎎/㎏), and at high-dose OHE group (300 ㎎/㎏), it was investigated similar to the normal group and the positive group.
7. OHE activate anti-diabetes pathway in liver of db/db mice.
Regulation of blood glucose is know that it regulated through insulin signaling pathway and glucose absorption. Phosphoinositide 3-kinase/phosphorylated protein kinase B (PI3K/p-Akt) and glucose transporter type 4 (Glut4) are important in the insulin signaling pathway. The decrease in blood sugar is observed to decrease the phosphorylation of Akt, increase the phosphorylation of AMPK, and increase the expression of Glut4.
To evaluate whether OHE lower blood glucose via regulating adenosine monophosphate-activated protein kinase (AMPK) signaling pathway and Glut4 expression, the relative protein levels of phospho-AMPK, phospho-Akt and Glut4 expression in liver were determined by western blot analysis. In Fig. 6, OHE showed increased phosphorylation of AMPK and expression of Glut4. However, the phosphorylation of Akt did not increase.
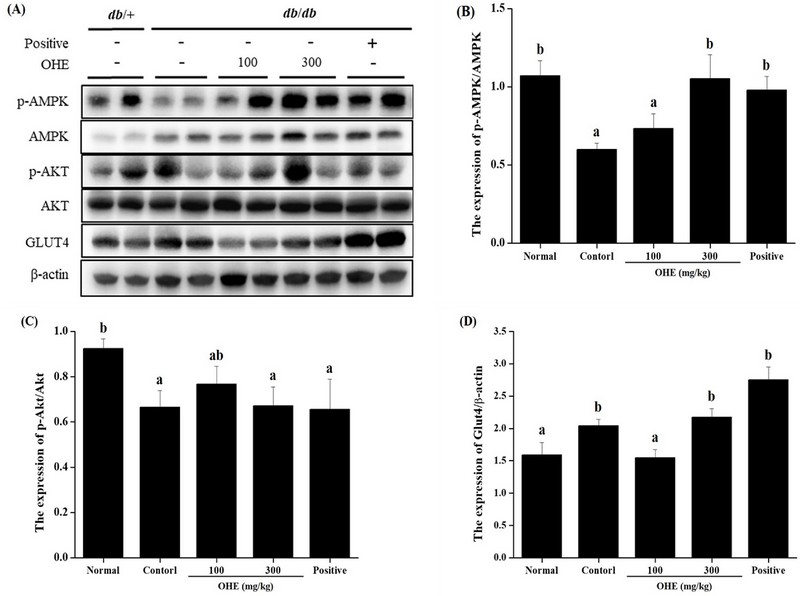
OHE increased anti-diabetes pathway in db/db mice. Mice received phosphate buffered saline (PBS), OHE (100, and 300 ㎎/㎏), and positive (metformin, 100 ㎎/㎏) once daily for 4 weeks. At the end of experiment, liver tissue was isolated from all experimental mice. (A) representative immunoblotting image, (B) the expression levels of phosphorylated adenosine monophosphate-activated protein kinase (phospho-AMPK). (C) the expression level of phospho-Akt. (D) the expression level of Glut4. Data are presented as the mean ± standard error (n = 7). Bars labeled with different superscripts have significantly different values at 5% using Duncan’s Multiple Range Tests (DMRT, p < 0.05).
DISCUSSION
O. humifusa is known to effective against adult diseases (such as hypertension, arthritis, and gastritis) because it contains polyphenols, flavonoids, and vitamin C.
In previous studies, O. humifusa or complex compound (cotained O. humifusa) has been reported to improve lipid metabolism and lower blood sugar in streptozotocin-induced diabetic rats (Shin et al., 2003; Hahm et al., 2011; Yoon, 2013). In this study, we investigated the potential anti-diabetic effects of OHE in db/db mice.
Previous studies have shown that oxidative stress resulting from increased ROS plays a role in inducing hyperglycemia, decreasing insulin secretion stimulated by glucose, and increasing cell death of pancreatic β cells (Ihara et al., 1999; Gurzov et al., 2014; Wang et al., 2014). α-glucosidase and α-amylase inhibition is an important treatment for hyperglycemia in diabetic patients (Casirola and Ferraris, 2006; Gao et al., 2013).
In this study, OHE was found to have strong antioxidant efficacy, and the effect on the α-glucosidase and α-amylase inhibition was investigated to determine the anti-diabetes efficacy (Fig. 1). As a result, it is explained that OHE has effective against diabetes. Additionally, the blood glucose decreased efficacy of OHE was evaluated with OGTT in ICR mice, and the results showed a significant decreased in blood glucose after 30 minutes after intake 2 g/㎖ glucose (Fig. 2). It proved to have excellent efficacy in reducing blood glucose after intake glucose.
In db/db mice, we show that the anti-diabetic effect and mechanism. Body weight and food intake did not change significantly by OHE compared to control (only PBS). And, it significantly inhibited the increased post-prandial blood glucose and fructosamine compared to PBS-treated db/db mice (Fig. 3C and D).
Insulin plays an pivotal role in glycogen synthesis and in enhancing glucose tolerance and inhibiting glucose production to maintain postprandial glucose levels within normal ranges (Bouche et al., 2004). Our result show that plasma insulin level was decreased by OHE in db/db mice (Fig. 3E). Additionally, HOMA-IR score decreased by OHE in db/db mice (Fig. 3F).
It is well known that serum lipid levels are elevated in diabetic patients (Howard, 1987). Therefore, significant adjustment of serum lipid levels in OHE-treated db/db mice may be encouraging to improve OHE insulin levels. Our results confirmed lipid improvement by significantly decreasing TG, TC, HDL-cholesterol, LDL-cholesterol, and VLDL-cholesterol levels in OHE group mice (Fig. 4).
These results suggest that db/db diabetes mice has anti-diabetes effects. Finally, the distribution of β cells through pancreatic H&E staining was investigated (Fig. 5).
Lastly, it was observed that the distribution of β cells in pancreatic tissue from normal and db/db mice was decreased compared to the normal group, and the distribution of β cells in the OHE groups and the positive group were increased (Fig. 5B).
AMPK, a cellular energy metabolism homeostasis regulator, is expressed in peripheral tissue such as liver and heart and Glut4 is carrier/transporter of glucose. In this study, we investigated whether OHE affects decrease of blood glucose level by regulation of AMPK signaling pathways and Glut4 expression in liver of db/db mice (Fig 6).
Glut4 is known to play an important role in glucose homeostasis and glucose influx to peripheral tissues. Glut4 is in the cell membrane and performs bioregulatory functions, when activated by insulin, its expression increases, and in a high blood glucose condition, it mediates glucose inflow into peripheral tissues to maintain glucose homeostasis in the body (Watson and Pessin, 2001). Our results showed increased of Glut4 expression and phosphorylation of AMPK by OHE in db/db mice (Fig 6. B and D). In this study, expression of Glut4 increased by OHE in db/db mice is thought to play an important role in the activity of Glut4 along with AMPK cell signaling involved in insulin-induced regulation of Glut4 expression and activity.
In conclusion, OHE showed anti-diabetes efficacy in in vitro studies (such as α-glucosidase and α-amylase inhibition experiments). And a decrease in blood glucose level, plasma lipid level and cholesterol levels was investigated in db/db mice. And it show that increased distribution of β cells in pancreatic tissue in db/db mice.
Finally, it has been verified for the activity of blood glucose drop and absorption mechanism in db/db mice. Therefore, as the anti-diabetes efficacy of OHE is proven, it is believed that it can help develop a diabetes treatment with fewer side effects using it.
Acknowledgments
This work was supported by the “Food Functionality Evaluation Program” under the Ministry of Agriculture, Food, and Rural Affairs and the “The Research Program” of the Korea Food Research Institute.
References
-
Bouche C, Serdy S, Kahn CR, and Goldfine AB. (2004). The cellular fate of glucose and its relevance in type 2 diabetes. Endocrine Reviews. 25:807-830.
[https://doi.org/10.1210/er.2003-0026]

-
Casirola DM and Ferraris RP (2006). α-Glucosidase inhibitors prevent diet-induced increases in intestinal sugar transport in diabetic mice. Metabolism. 55:832-841.
[https://doi.org/10.1016/j.metabol.2006.02.011]

-
Chavez-Santoscoy RA., Gutierrez-Uribe JA, and Serna-Saldivar SO. (2009). Phenolic composition, antioxidant capacity and in vitro cancer cell cytotoxicity of nine prickly pear (Opuntia spp.) juices. Plant Foods for Human Nutrition. 64:146-152.
[https://doi.org/10.1007/s11130-009-0117-0]

-
Cho JY, Park SC, Kim TW, Kim KS, Song JC, Lee HM, Sung HJ, Rhee MH, Kim SK, Park HJ, Song YB, Yoo ES and Lee CH. (2006). Radical scavenging and anti-inflammatory activity of extracts from Opuntia humifusa Raf. Journal of Pharmacy and Pharmacology. 58:113-119.
[https://doi.org/10.1211/jpp.58.1.0014]

-
Dinis TC, Maderia VM and Almeida LM. (1994). Action of phenolic derivatives(acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Archives of Biochemistry and Biophysics. 315:161-169.
[https://doi.org/10.1006/abbi.1994.1485]

-
du Toit R, Volsteedt Y and Apostolides Z. (2001). Comparison of the antioxidant content of fruits, vegetables and teas measured as vitamin C equivalents. Toxicology. 166:63-69.
[https://doi.org/10.1016/S0300-483X(01)00446-2]

-
Gao J, Xu P, Wang Y, Wang Y, and Hochstetter D. (2013). Combined effects of green tea extracts, green tea polyphenols or epigallocatechin gallate with acarbose on inhibition against alpha-amylase and alpha-glucosidase in vitro. Molecules. 18:11614-11623.
[https://doi.org/10.3390/molecules180911614]

-
Gao T, Ma S, Song J, Bi H, and Tao Y. (2011). Antioxidant and immunological activities of water-soluble polysaccharides from Aconitum kusnezoffii Reichb. International Journal of Biological Macromolecules. 49:580-586.
[https://doi.org/10.1016/j.ijbiomac.2011.06.017]

-
Goldstein BJ. (2002). Insulin resistance as the core defect in type 2 diabetes mellitus. American Journal of Cardiology. 90:3G-10G.
[https://doi.org/10.1016/S0002-9149(02)02553-5]

-
Gurzov EN, Tran M, Fernandez-Rojo MA, Merry TL, Zhang X, Xu Y, Fukushima A, Waters MJ, Watt MJ, Andrikopoulos S, Nell BG and Tiganis T. (2014). Hepatic oxidative stress promotes insulin-STAT-5 signaling and obesity by inactivating protein tyrosine phosphatase N2. Cell Metabolism. 20:85-102.
[https://doi.org/10.1016/j.cmet.2014.05.011]

-
Hahm SW, Park J and Son YS. (2011). Opuntia humifusa stems lower blood glucose and cholesterol levels in streptozotocin-induced diabetic rats. Nutrition Research. 31:479-487.
[https://doi.org/10.1016/j.nutres.2011.05.002]

-
Han AR, Lim TG, Song YR, Jang M, Rhee YK, Hong HD, Kim MH, Kim HJ and Cho WC (2018). Inhibitory effect of Opuntia humifusa fruit water extract on solar ultraviolet-induced MMP-1 expression. International Journal of Molecular Sciences. 19:2503. https://www.mdpi.com/1422-0067/19/9/2503, (cited by 2024 July 15).
[https://doi.org/10.3390/ijms19092503]

-
Howard BV. (1987). Lipoprotein metabolism in diabetes mellitus. Journal of Lipid Research. 28:613-628.
[https://doi.org/10.1016/S0022-2275(20)38659-4]

-
Ihara Y, Toyokuni S, Uchida K, Odaka H, Tanaka T, Ikeda H, Hiai H, Seino Y and Yamada Y. (1999). Hyperglycemia causes oxidative stress in pancreatic β-cells of GK rats, a model of type 2 diabetes. Diabetes. 48:927-932.
[https://doi.org/10.2337/diabetes.48.4.927]

-
Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, Taskinen R and Groop L. (2001). Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 24:683-689.
[https://doi.org/10.2337/diacare.24.4.683]

-
Kang JY, Park JH, Choi SH, Igawa S and Song YJ. (2012). Opuntia humifusa supplementation increased bone density by regulating parathyroid hormone and osteocalcin in male growing rats. International Journal of Molecular Sciences. 13:6747-6756.
[https://doi.org/10.3390/ijms13066747]

-
Kang YR, Lee HY, Kim JH, Moon DI, Seo MY, Park SH, Choi KH, Kim CR, Kim SH, Oh JH, Cho SW, Kim SY, Kim MG, Chae SW, Kim O and Oh HG. (2012). Anti-obesity and anti-diabetic effects of Yerba Mate (Ilex paraguariensis) in C57BL/6J mice fed a high-fat diet. Laboratory Animal Research. 28:23-29.
[https://doi.org/10.5625/lar.2012.28.1.23]

-
Koh LW, Wong LL, Loo YY, Kasapis, S and Huang D. (2010). Evaluation of different teas against starch digestibility by mammalian glycosidases. Journal of Agricultural and Food Chemistry. 58:148-154.
[https://doi.org/10.1021/jf903011g]

-
Kumar S, Narwal S, Kumr V and Prakash O. (2011). alpha-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacognosy Reviews. 5:19-29.
[https://doi.org/10.4103/0973-7847.79096]

-
Lee EJ, Choi H, Yoon WC, Kim YS, Song B, Lee MH, Park B, Lee SH, Choi JH and Park SY. (2021). Anti-diabetic and lipid profile effect of Astragalus membranaceus(Fisch.) Bunge fermented by Aspergillus awamori in db/db mice. Korean Journal of Medicinal Crop Science. 29:263-272.
[https://doi.org/10.7783/KJMCS.2021.29.4.263]

-
Liu F, Kim J, Li Y, Liu X, Li J and Chen X. (2001). An extract of Lagerstroemia speciosa L. has insulin-like glucose uptake-stimulatory and adipocyte differentiation inhibitory activities in 3T3-L1 cells. The Journal of Nutrition. 131:2242-2247.
[https://doi.org/10.1093/jn/131.9.2242]

-
Ngoc LTN, Moon JY, Lee YC. (2023). Beneficial effects of Opuntia humifusa(Korean Cheonnyuncho) on human health based on antioxidant properties: Systematic review and meta-analysis. Antioxidants. 12:174. https://www.mdpi.com/2076-3921/12/1/174, (cited by 2024 July 15)
[https://doi.org/10.3390/antiox12010174]

- Shin JE, Han MJ, Lee IK, Moon YI and Kim DH. (2003). Hypoglycemic activity of Opuntia ficus-indica var. sabotan on alloxan-or streptozotocin-induced diabetic mice. Korean Journal of Pharmacognosy. 34:75-79.
-
Wang X, Zhang W, Chen H, Liao N, Wang Z, Zhang X and Hai C. (2014). High selenium impairs hepatic insulin sensitivity through opposite regulation of ROS. Toxicology Letters. 224:16-23.
[https://doi.org/10.1016/j.toxlet.2013.10.005]

-
Watson RT, and Pessin JE. (2001) Intracellular organization of insulin signaling and GLUT4 translocation. Recent Progress in Hormone Research. 56:175-193.
[https://doi.org/10.1210/rp.56.1.175]

-
Yang EI, Lee CH, Che DN, Jang SI and Kim YS. (2019). Biological activities of water-soluble polysaccharides from Opuntia humifusa stem in high-fat-diet-fed mice. Jouranl of Food Biochemistry. 43:e12806. https://onlinelibrary.wiley.com/doi/full/10.1111/jfbc.12806, (cited by 2024 May 05).
[https://doi.org/10.1111/jfbc.12806]

-
Yoo CY, Son HU, Fanar A, Choi HJ, Alam MB, and Lee SH. (2020). Opuntia humifusa aqueous extract alleviates ethanol-induced gastric ulcer in a mouse model. Asian Pacific Journal of Tropical Biomedicine.10:403-410. https://journals.lww.com/aptb/fulltext/2020/10090/opuntia_humifusa_aqueous_extract_alleviates.3.aspx#, (cited by 2024 July 15).
[https://doi.org/10.4103/2221-1691.290131]

-
Yoon JA. (2013). Effects of Opuntia ficus-indica complex on lipid metabolism in streptozotocin-induced diabetic rats. Korean Journal of Food and Nutrition, 26:526-534.
[https://doi.org/10.9799/ksfan.2013.26.3.526]
