
Effect of Light Intensity on Growth and Volatile Compound Content of Valeriana fauriei a Walk-In Growth Chamber
This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Valeriana fauriei infusion is provided as a sedative and to treat nervous system disorders. The roots and rhizomes, known for their bitter taste and distinct aroma, serve therapeutic purposes, whereas the upper tissues are consumed as food. Light serves as an energy source and provides developmental signals for plants, influencing crop physiology and biochemistry. We examined the effect of various light intensities (100, 400, and 800 μmol·m−2·s−1) on V. fauriei growth and development.
Higher light intensities reduced plant height, but increased biomass and number of branches. Photosynthetic parameters were higher at 100 μmol·m−2·s−1, whereas photodamage under higher light intensities reduced chlorophyll content. Using gas chromatography/mass spectrometry, we identified isovaleric acid as the predominant volatile compound, and significant levels of bornyl acetate and valeric acid, in V. fauriei. Changes in light intensity also influenced volatile compound levels, with optimal production of bornyl acetate and isovaleric acid observed at 400 μmol·m−2·s−1, while valeric acid production reached its maximum at 800 μmol·m−2·s−1.
Adjusting ambient light conditions can effectively optimize production of desirable compounds. Specifically, a light intensity of 400 μmol·m−2·s−1 was established as optimal for enhancing plant biomass and total volatile compounds.
Keywords:
Valeriana fauriei, Light Intensity, Growth Characteristics, Chlorophyll, Volatile CompoundINTRODUCTION
Valeriana fauriei L. is a perennial medicinal plants that belong to the Valerianaceae family, and their roots and rhizomes are utilized for therapeutic purposes. These roots and rhizomes have a slightly bitter taste and a noticeable scent.
Currently, approximately 200 Valeriana species have been recognized, with the majority of wild or cultivated species distributed worldwide, such as Europe, United States, and parts of Northern Asia (Li et al., 2022). In Korea, approximately eight Valeriana species are indigenous, typically growing in shaded, humid habits such as forested areas, riverbanks, and stream embankments (Jin et al., 2007).
For centuries, its roots have been used in sedative infusions and to treat disorders of the nervous system in both homeopathic and allopathic treatments, while the aerial parts have been consumed as food (Wang et al., 2010).
According to earlier research, the main constituents of Valeriana species include camphene, β-terpineol, azulene, geraniol, borneol, and β-caryophyllene, as well as a number of ketones and esters (such as bornyl acetate, bronyl-formiate, bornyl-isovalerate, eugenyl-isovalerate, and isoeugenyl) and valeric and isovaleric acids (Wang et al., 2017).
Of these, valeric acid, also known as pentanoic acid, is a key active compounds in Valeriana species, reported to have therapeutic effects on conditions such as insomnia and seizures, with recent studies highlighting its potential to enhance cancer immunity (Han et al., 2020).
Isovaleric acid, characterized by its distinctive unpleasant odor, is also suggested to be the primary anticonvulsant component within Valeriana plant extracts (Patočka and Jakl. 2010).
Bornyl acetate, a bicyclic monoterpene found in various natural sources, is a typical volatile compound with significant medicinal value, demonstrating antimicrobial, anticancer, anti-inflammatory, and anti-abortive properties (Zhao et al., 2023).
Light serve as both an energy source and a developmental signal for plants, while also acting as a modulator of stress responses (Roeber et al., 2021). It also significantly influences the biosynthesis and accumulation of various secondary metabolites, which are vital for crop quality (Thoma et al., 2020).
In horticulture, three primary variables are essential when considering light requirements: light quality, light intensity, and photoperiodism. Light quality involves adjusting spectral compositions, either with targeted narrow peaks or broad, continuous spectra, achievable with LED systems, high-pressure sodium lamps, and other light sources.
Light intensity has advanced with modern lighting capable of exceeding the light saturation point, where the net photosynthetic rate per unit leaf area reaches its maximum, which in leafy greens typically occurs below 1000 μmol·m−2·s−1 (Bian et al., 2015).
Photoperiodism considers not only day-night cycles but also allows for additional supplemental lighting periods, providing a vast range of possible light management (Thoma et al., 2020).
Light intensity is one of the most crucial environmental factors affecting crop physiology and biochemistry (Yang et al., 2018). In previous study, light intensity affects a number of physiological characteristics in plants, such as transpiration, stomatal conductance, photosynthetic rates, stem diameter, and the accumulation of dry matter throughout the plant, including the roots, stems, leaves, and the entire plant (Feng et al., 2019).
Plant requires a specific range of light intensity for optimal growth, as deviations-whether higher or lower-can suppress photosynthesis. Lower light intensities or shading, such as in intercropping systems, reduce leaf growth, result in thinner leaves with smaller surface area, thinner palisade tissues, and diminished chlorophyll content, thereby decreasing light-harvesting efficiency.
Conversely, excessive light intensity can disrupt the balance between energy supply and consumption. Studies have shown that plants exposed to varying light intensities exhibit differences in photosynthetic pigment composition, electron carriers, chloroplast ultrastructure, and photosynthetic rates (Shafiq et al., 2021).
Light has also a direct and indirect impact on the production of bioactive compounds in medicinally important species, which are closely related to the synthesis of secondary metabolites. For instance, light is essential in the in vitro culture of Lippia gracillis, as it influence both grown and volatile compound production. Lower light intensity improves the accumulation of photosynthetic pigments in this plant. In addition, it had a considerable impact on the volatile constituent profile, affecting both quantitative and qualitative aspects (Lazzarini et al., 2018).
The roots of V. fauriei have long been used as a medicinal herb, while its shoots, such as young leaves, have traditionally been consumed as food. Building on this traditional usage, the study examines the effect of various light intensities on growth, photosynthetic parameters, chlorophyll content and the quantitative levels of volatile compounds in roots and shoots of V. fauriei to determine optimal light conditions, cultivated in a walk-in growth chamber.
MATERIALS AND METHODS
1. Plant material
V. fauriei seeds were observed from Eumseong, Chung-cheongbuk-do, Korea and germinated in moist rock-wool cubes. A lightweight horticultural universal soil (Baroker, Seoulbio. Co., Ltd., Eumseong, Korea) was evenly filled into pots and adequately moistened prior to transplanting V. fauriei seedlings.
In the experiment, seedlings with three expanded true leaves were transplanted into individual pots (one plant per pot) and cultivated in a walk-in growth chamber at the Eumseong, Chungcheongbuk-do, Korea. A total of 5 pots were used for each treatment. The internal environmental conditions of the growth chamber were set at 23 ± 2℃, 60% relative humidity, with a 16 hr light / 8 hr dark photoperiod. Three light intensities (photosynthetic photon flux density, PPFD) were used: 100, 400, and 800 μmol·m−2·s−1 (100L, 400L, and 800L). In addition, Irrigation was performed once every three days to prevent the pots from drying out. These conditions were maintained for 12 weeks. Plant height, branch count, and Soil Plant Analysis Development (SPAD) value were tracked on a weekly basis.
At harvest, the fresh weight of whole plant and root was determined. The roots and shoots were subjected to oven drying at 50℃ for three days, then dried weight was observed. All dried materials were subsequently ground into fine powder for further analyses.
2. Pigment content measurement
Pigments, including chlorophyll a and b, were extracted from V. fauriei grown under different light intensities, following a previously reported method (Lichtenthaler and Buschmann, 2001).
Depending on the solvent system employed, absorbance at 665.2, and 652.4 ㎚ was measured in order to quantify the pigments. For extraction, 20 ㎎ of the sample was dissolved in 90% methanol, and solvent-specific equations were used to determine pigment concentrations.
Chlorophyll a (㎍·㎖–1) = (16.82 × A665.2) – (9.28 × A652.4)
Chlorophyll b (㎍·㎖–1) = (36.92 × A652.4) – (16.54 × A665.2)
3. Gas chromatography/mass spectrometry (GC-MS) analysis
One gram of the powder was extracted with 10 ㎖ of n-hexane through sonication at 40℃ for 30 min. The upper aqueous phase was isolated via centrifugation at 3,500 rpm at 4℃ for 10 min, and this process was repeated twice more. All collected aqueous phases were evaporated to dryness using a SpeedVac vacuum concentrator (SPD210P1, Thermo Fisher Scientific, Waltham, MA, USA). The dried extract was reconstituted in n-hexane to a final concentration of 10,000 ppm, followed by filtration with a 0.45 ㎛ syringe filter, and utilized for subsequent analyses.
GC-MS analysis was performed using a gas chromatography-mass selective detector (Perkin Elmer, Waltham, MA, USA) equipped with an HP-INNOWax capillary column (60 m × 0.25 ㎜ i.d. × 0.25 ㎛, Agilent, Santa Clara, CA, USA) and a flame ionization detector (Clarus 690, Perkin Elmer, Waltham, MA, USA) at the Gyeongnam Bio and Anti-aging Core Facility Center (Changwon, Korea).
The injector was maintained at 250℃, with helium as the carrier gas at a flow rate of 1 ㎖·min-1. The GC oven was programmed to start at 70℃ for an initial 5 min, followed by a temperature ramp to 200℃ at 10℃․min-1, and sustained at 200℃ for an additional 5 min. With an ion source temperature of 200℃, a trap current of 250 ㎂, and an ionization voltage of 70 eV, mass spectra were acquired. Chromatograms of total ion current monitored across the 40 amu - 450 amu range.
An external standard method with a calibration curve was employed for quantification. Bornyl acetate (Cat. No. B55203) and valeric acid (Cat. No. 75054), purchased from Sigma-Aldrich (St. Louis, MO, USA), were used to establish calibration curves over a concentration range of 10 to 250 ㎍·㎖-1. The regression equations for bornyl acetate and valeric acid were y = 267287x – 921098 (r2 = 0.9994) and y = 124374x – 2E + 06 (r2 = 0.9997), respectively.
In the absence of a standard compound for quantitative analysis, various strategies have been developed, including the use of structurally similar compounds (Kruve A, 2020). This approach was applied for the quantification of isovaleric acid in this study. By considering the structural and chemical similarities between the standard compound and the target analyte, the method ensures the validity and reliability of the results.
Specifically, valeric acid and isovaleric acid share the same chemical formula (C5H10O2) and have an identical exact mass of 102.13 g·mol−1. Based on these strong similarities, the calibration curve of valeric acid was employed to quantify isovaleric acid.
4. Statistical analysis
A one-way analysis of variance (ANOVA) was conducted to evaluate significant differences among treatments under varying light intensities, with Tukey’s HSD test applied for post-hoc analysis at a significance level of 5% using SPSS 26.0 (p < 0.05, SPSS Inc., Chicago, IL, USA).
Growth parameters were measured from five biological replicates, while pigment and volatile compound measurements were based on three technical replicates. Data in all tables and figures are presented as the means ± standard deviation (SD).
RESULTS AND DISCUSSION
1. Growth Characteristics
V. fauriei plants were cultivated under varying light intensity conditions for 12 weeks after transplanting in a walk-in growth chamber, with weekly measurements taken for plant height and number of branch. From the early growth stages until the 8 week following transplantation, no significant differences were observed between treatments for plant height (Fig. 1A).
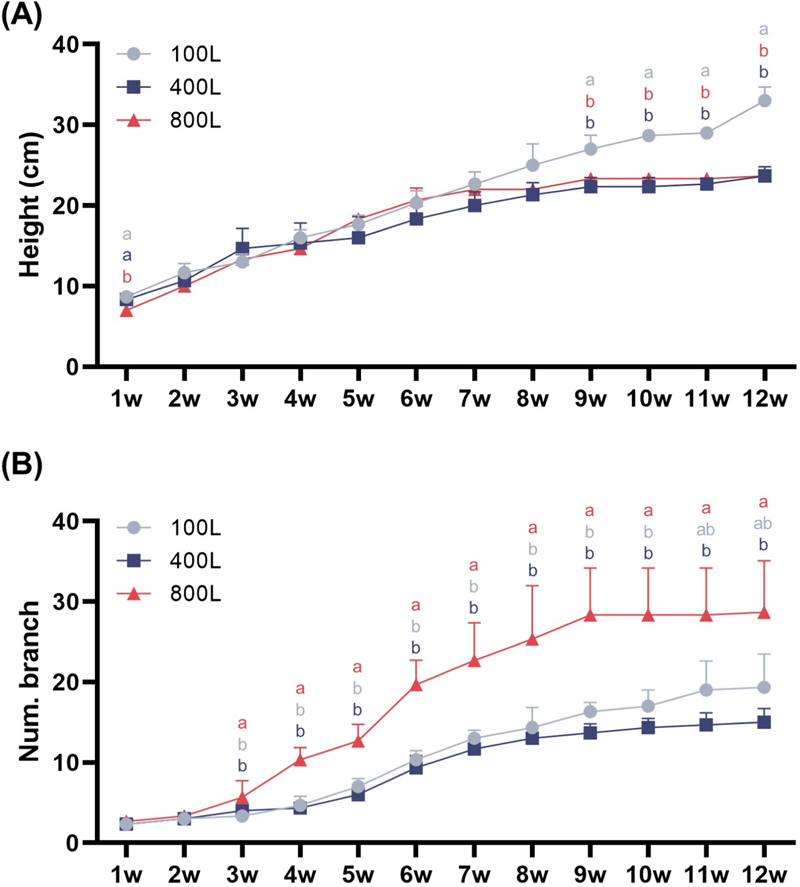
Growth parameters of V. fauriei plants under various light intensity treatments including 100L, 400L, and 800L.Plant height (A) and the number of branches (B) were evaluated weekly following transplantation. Three levels of light intensity were used: 100, 400, and 800 μmol·m−2·s−1, designated as 100L, 400L, and 800L. Closed markers and vertical bars represent means ± standard deviation (n = 5). The x-axis represents weeks after transplanting. Significant differences between treatments, as determined by a one-way ANOVA and Tukey’s HSD test at 5%, are indicated by different letters (*p < 0.05).
In the later growth stages, plants subjected to the lower light intensity of 100L showed the greatest plant height, reaching approximately 33 ㎝. However, plant height growth stopped around 9 weeks under higher light intensities of 400L and 800L, with heights of roughly 23 cm, respectively. In Fig. 1B, the measurement of branch number revealed that V. fauriei treated with 800L had highly more branches throughout the cultivation period compared to the other treatments. In contrast, there was no notable difference in branch number between 100L and 400L.
After cultivation under different light intensities for 12 weeks, the photographs of V. fauriei obtained post-harvest are presented in Fig. 2. It clearly showed that the plants treated with 100L display significantly greater plant height. Furthermore, as the intensity of light increases, an observable increase in root biomass can be noted.

Photographs of V. fauriei plants under various light intensity treatments including 100L (A), 400L (B), and 800L (C), taken 12 weeks after transplantation.Three levels of light intensity were used: 100, 400, and 800 μmol·m−2·s−1, designated as 100L, 400L, and 800L.
The fresh weight of the whole plant and roots, as well as the dried weight of the roots and shoots were observed and indicated in Fig. 3. The 800L treatment exhibited the most notable increase in all weight-related measurements, followed by 400L and then 100L. In detail, the 800L treatment yielded the highest value at 151 g, followed by 400L at 103 g, and 100L at 86 g for fresh weight of whole plant (Fig. 3A).
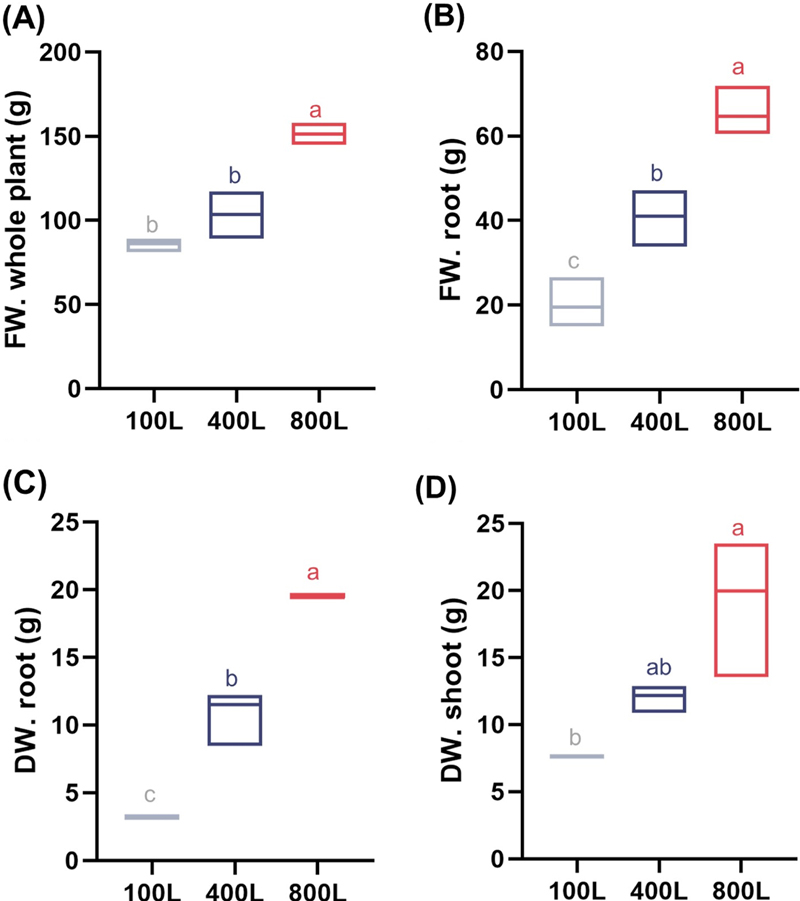
Weight parameters of V. fauriei plants under various light intensity treatments including 100L, 400L, and 800L.Fresh weight of the whole plant (A) and root (B), as well as dried weight of the root (C) and shoot (D) were assessed at 12 weeks after transplantation (n = 5). Three levels of light intensity were used: 100, 400, and 800 μmol·m−2·s−1, designated as 100L, 400L, and 800L. In the box plots, the lower and upper fences represent the minimum and maximum values, while the mean is depicted between them. The x-axis represents the various light intensities used in this study: 100L, 400L, and 800L. Significant differences between treatments, as determined by a one-way ANOVA and Tukey’s HSD test at 5%, are indicated by different letters (*p < 0.05). FW, fresh weight; DW, dried weight.
In addition, the 800L treatment produced the highest root fresh and dried weight values, evaluating 64 g and 19 g, respectively (Fig. 3B and 3C). It indicates that higher light intensity improves root development in V. fauriei.
Similar trends were apparent in the dried weight of the shoots, which were 19 g, 12 g, and 7 g for the 800L, 400L, and 100L treatments, for each (Fig. 3D). These findings imply the increased light intensity successfully encouraged a rise in plant biomass.
A certain amount of variability in crop morphology allows for adaptive responses to changing environmental conditions (Gong et al., 2015). Improving crop yields can be achieved through the use of intercropping systems and higher plant populations. Different species and cultivars require specific light levels for optimal growth, with an ideal range of 200 μmol·m−2·s−1 – 300 μmol·m−2·s−1 for leafy vegetables under controlled conditions. In cultivation, light intensity should be determined by the physiological demands and maintained within an appropriate range (Bian et al., 2015). However, reducing light levels usually makes these techniques ineffective. Up to date, few studies have examined how plant morphology is affected by variations in light intensity.
Previous study revealed that increased light reduced hypocotyl length, leaf petiole angle, and plant height with light intensities in the range of 100 to 500 μmol·m−2·s−1. Conversely, biomass, root-to-shoot ratio, and stem diameter were higher, with peak values at 400 and 500 μmol·m−2·s−1 (Feng et al., 2019).
These findings are consistent with the results of this study. Under higher light intensity conditions, plant height was reduced, whereas the number of branches and all weight-related parameters were maximized under the highest intensity treatment of 800L.
The assessment of the root-to-shoot ratio revealed comparable findings, with ratio of 0.39, 0.80, 1.03 for the 100L, 400L, 800L treatments, respectively (Table 1).
These findings suggest that increased light intensity significantly improves root development. Comparable findings were observed in Lippia gracilis and Hyptis suaveolens, where root length, as well as both root and total weight, increased with higher light intensity (Lazzarini et al., 2018; Bankole et al., 2022).
Auxin levels may be attributed to the improvement in root length given that there is evidence that light intensity has an impact on the synthesis and transport of hormones, leading to consequently influence root formation (Zhang et al., 2009).
2. Chlorophyll content and the SPAD value
Throughout the cultivation period, the SPAD values of V. fauriei were determined on a weekly basis and the results are shown in Fig. 4A.
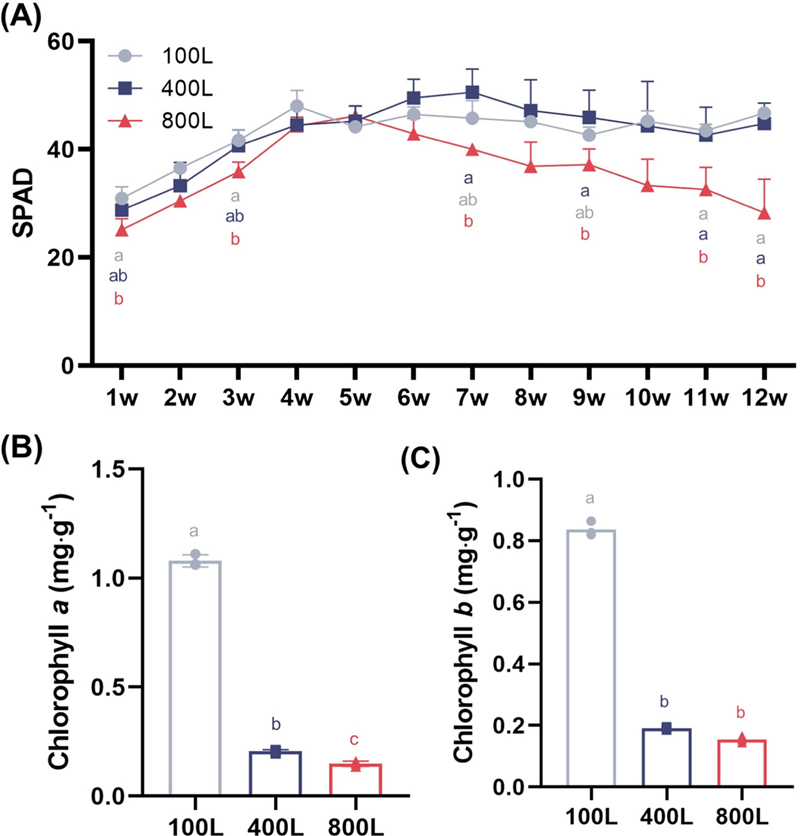
SPAD value (A) obtained weekly following transplantation and chlorophyll a (B) and chlorophyll b (C) contents of shoots in V. fauriei plants cultivated under different light intensities.The x-axis represents weeks after transplanting for SPAD values and the light intensities (100L, 400L, and 800L) for chlorophyll content. Three levels of light intensity were used: 100, 400, and 800 μmol·m−2·s−1, designated as 100L, 400L, and 800L. Significant differences between treatments, as determined by a one-way ANOVA and Tukey’s HSD test at 5%, are indicated by different letters (*p < 0.05).
The SPAD value revealed a trend opposite to the growth parameters: under the highest light intensity (800L), the SPAD values initially increased until 5 weeks after transplanting, at which point they began to decrease and eventually reached their lowest levels among the various treatments. In contrast, it remained relatively stable throughout the cultivation period under the 100L and 400L treatments, showing no significant differences from one another.
As shown in Fig. 2, the greenish color of the V. fauriei shoots visibly diminishes with higher levels of light intensity. Additionally, the UV-Vis spectrophotometric method was used to assess the chlorophylls content present in the shoots. Chlorophyll a and b contents were significantly accumulated under the 100L treatment, and as light intensity increased, chlorophyll content decreased, which is consistent with the SPAD value results (Fig. 4B and 4C).
Optimal light intensity promotes photosynthesis and increases dry matter accumulation, while excessive light can cause photodamage, significantly impacting plant growth and development (Bian et al., 2015).
Under strong visible light, plants experience a reduction in photosynthetic rate due to processes such as photoinhibition, photo-oxidation, photoinactivation, photolability, solarization, and photodynamic reaction. Photo-inhibition refers to the reduction in a plant’s photosynthetic capacity caused by excessive light exposure, which frequently results by a decrease in pigment concentration (Powles, 1984).
Moreover, high light stress adversely impacts essential photosynthetic processes, including the chlorophyll/carotenoid ratio, electron transport flux, photochemical efficiency, and the maximum quantum efficiency of photosystem II (Sharma et al., 2020). Moreover, chlorophyll b is degraded by high light stress through an isozyme called chlorophyll b reductase (Sato et al., 2015).
Therefore, the observed reduction in chlorophyll a and b content with increasing light intensity from 100L to 800L treatment is likely a consequence of photodamage resulting from stress induced by extremely high light conditions.
3. Volatile compounds and their productivity
The root and shoot were analyzed using GC-MS analysis to quantify bornyl acetate, isovaleric acid, and valeric acid, which are primary volatile compounds in V. fauriei (Table 2 and Fig. 5).

The contents of major volatile compounds including bornyl acetate, isovaleric acid, and valeric acid in roots and shoots of V. fauriei treated with different light intensities.
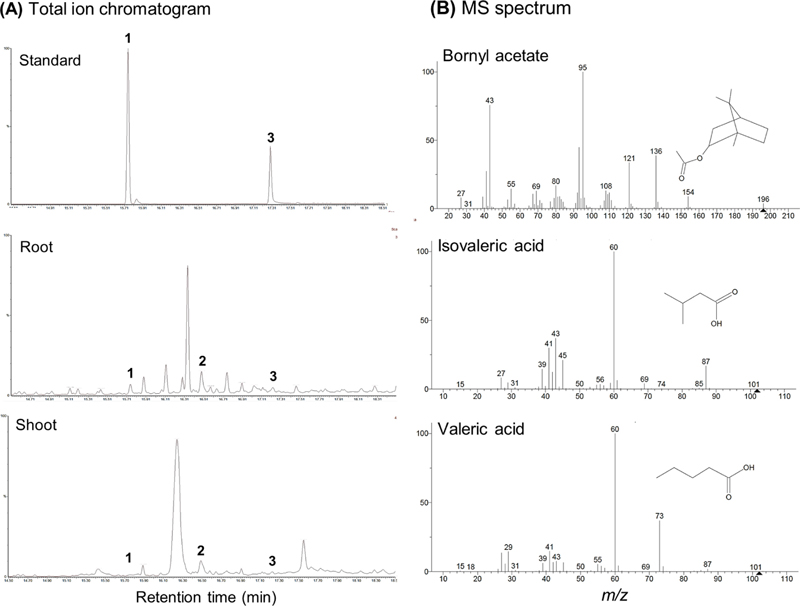
(A) Representative total ion chromatograms of standard, root, and shoot of V. fauriei and (B) MS spectrum of volatile compounds including bornyl acetate, isovaleric acid, and valeric acid obtained from GC-MS analysis.1: Bornyl acetate (15.75 min), 2: Isovaleric acid (16.48 min), and 3: Valeric acid (17.29 min).
Bornyl acetate accumulated most in the roots under the 400L treatment, reaching 0.084 ㎎·g-1 dry weight (DW), while the 100L and 800L treatments resulted in lower levels at 0.032 and 0.025 ㎎·g-1․DW, respectively.
In contrast, there was no significant variation in bornyl acetate content observed in the shoots across three light treatments. For isovaleric acid, the concentrations in the roots exhibited a decline with increasing light intensity, obtained at 0.817 ㎎·g-1․DW, 0.472 ㎎·g-1․DW, and 0.266 ㎎·g-1․DW for 100L, 400L, 800L treatments, respectively.
In the shoots, isovaleric acid levels were quantified at 0.255 ㎎·g-1․DW and 0.288 ㎎·g-1․DW for the 100L and 400L treatments, respectively, while no detectable amount was found under the 800 L treatment.
For valeric acid, its levels in the shoots increased as light intensity rose from 100L to 400L but showed a declining trend at 800L, measured at 0.057 ㎎·g-1․DW, 0.072 ㎎·g-1․DW, 0.068 ㎎·g-1․DW, respectively. Similarly, it is highly likely that isovaleric acid, an isoquinoline alkaloid, followed the same pattern, explaining its absence at 800L.
Valeric acid concentrations in the roots also demonstrated a declining tread with increased light intensity, measured at 0.097 ㎎·g-1․DW, 0.072 ㎎·g-1․DW, 0.068 ㎎·g-1․DW for the 100L, 400L, and 800L treatments, respectively. However, no significant differences were found among the treatments.
These findings suggest that high light intensity induces stress in plants, leading to photoinhibition that reduces photosynthetic capacity and damages synthetic mechanisms. In this study, such stress likely negatively impacted the biosynthesis of volatile compounds.
The total productivity of the three major volatile compounds in both roots and shoots is presented in Fig. 6.
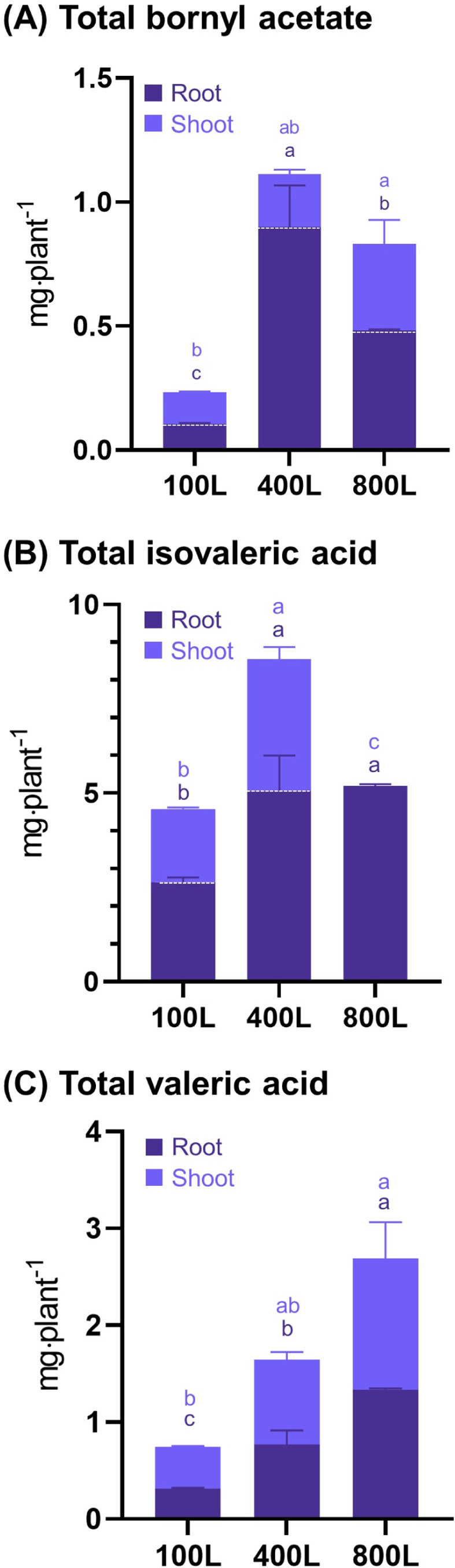
Total concentrations (㎎·plant-1) of bornyl acetate (A), isovaleric acid (B), and valeric acid (C) in the roots and shoots of V. fauriei under various light intensity treatments.The x-axis represents the various light intensities used in this study: 100L, 400L, and 800L. Significant differences between treatments, as determined by a one-way ANOVA and Tukey’s HSD test at 5%, are indicated by different letters (*p < 0.05).
The total bornyl acetate content per individual plant was indicated to be highest in the roots, with a concentration of 0.89 ㎎·plant-1 under the 400L treatment, followed by 0.48 ㎎·plant-1 at 800L and 0.01 ㎎․plant-1 at 100L (Fig. 6A).
In the shoots, the highest accumulation was observed at 0.34 ㎎·plant-1 under 800L treatment, followed by 0.21 ㎎·plant-1 at 400L and 0.12 ㎎·plant-1 at 100L.
Increased light intensities resulted in a significantly higher total accumulation of isovaleric acid in the roots; concentration were 5.05 and 5.18 ㎎·plant-1 for the 400L and 800L treatments, respectively (Fig. 6B).
By contrast, the accumulation of approximately 2.63 ㎎·plant-1, which is nearly half that observed under the higher light conditions, was substantially reduced by the lower light intensity treatment at 100L. Isovaleric acid concentrations in shoots were determined at 3.49 ㎎·plant-1 for 400L and 1.97 ㎎·plant-1 for 100L treatments.
Notably, isovaleric acid emerged as the primary volatile compounds among the three major components analyzed in this study, indicating its significant presence in V. fauriei. Total valeric acid was found to accumulate in both roots and shoots that followed the light intensity gradient of 800L, 400L, and 100L (Fig. 6C).
Specifically, in the roots, total valeric acid concentrations were 1.33 ㎎·plant-1, 0.77 ㎎·plant-1, and 0.31 ㎎·plant-1 for the 800L, 400L, and 100L treatments, respectively, while in the shoots, the values were 1.35 ㎎·plant-1, 0.87 ㎎·plant-1, and 0.43 ㎎·plant-1 for the same treatments.
According to the results of this study, dried weight of roots was measured at an average of 10.72 g and 19.50 g under 400L and 800L, respectively (Fig. 3C). Based solely on biomass productivity, 800L could be considered the optimal light intensity. However, as the roots of V. fauriei are utilized as the medicinal herb, the productivity of its key bioactive compounds, such as valeric acid, isovaleric acid, and bornyl acetate, is also an essential criterion.
Analysis of compound content per gram of root revealed that bornyl acetate was highest at 400L, while valeric acid and isovaleric acid showed a decreasing trend within increasing light intensity (Table 2). When extrapolated to total root productivity, bornyl acetate was most effectively produced at 400L. In contrast, isovaleric acid showed similar productivity at 400 and 800L, while valeric acid was highest at 800L (Fig. 6).
Considering the potential of light stress reactions under high light intensity in controlled environments and the economic feasibility, 400L was identified as the optimal light intensity for cultivation.
Valeriana species consist of over 800 compounds, which belong to a variety of classes including iridoids, lignans, flavonoids, sesquiterpenoids, alkaloids, essential oils and others (Li et al., 2022).
Despite the extensive use of roots and rhizomes from Valeriana species in medicine and various industrial applications, only a limited number of species have been thoroughly investigated in terms of their medicinal properties, biochemical profiles, or clinical applications. For example, the root of V. officinalis contains approximately 2% volatile oil, predominantly comprising bornyl acetate, a key monoterpene.
Additional compounds commonly found in the oil include valerenic acid, valeric acid, isovaleric acid, along with various monoterpenes and sesquiterpenes. Notably, isovaleric acid has been shown to possess anticonvulsant and sedative properties (Dyayiya et al., 2016).
In this study, we identified bornyl acetate, isovaleric acid, and valeric acid, demonstrating that isovaleric acid is the predominant compound in V. fauriei.
The light intensity can also change the volatile compound production of V. fauriei. According to our results, the three detected volatile compounds accumulated most abundantly in both roots and shoots under 400L treatment, which also positively influenced their entire plant productivity.
Previous research highlights that response in volatile compound production to light sources are highly species-dependent. For example, in Plectranthus amboinicus, lower light intensities enhanced carvacrol levels, while in L. gracilis, reduced light intensity led to a decrease in carvacrol content (Lazzarini et al., 2018; Silva et al., 2017).
Furthermore, research has demonstrated that variations in light quality and intensity affect the amount and constitute of volatile compounds from Achilea millefolium (Alvarenga et al., 2015).
In high-tech greenhouse, such as those used in vertical agriculture, artificial lighting serves both as assimilative and a regulatory role. In the assimilative function, artificial light optimizes photosynthetic efficiency under conditions of low solar radiation, such as at high latitudes or during winter months, thereby enhancing plant production. Meanwhile, the regulatory role involves guiding plant growth and development by inducing morphological changes (e.g., branch elongation observed in this study) or promoting the synthesis and accumulation of metabolites, enabling plants to adapt to adverse environmental conditions and enhancing both their fitness and the nutraceutical properties of their products (Cavallaro and Muleo, 2022).
In conclusion, the light intensity influenced the growth of plants, biomass, photosynthetic pigments, and the volatile compounds production of V. fauriei cultivated in walk-in growth chamber.
The higher light intensities of 400L and 800L resulted in reduced plant height while increasing the number of branches and overall biomass. In accordance with the SPAD value results, chlorophyll a and b levels were significantly higher under the 100L treatment, and the chlorophyll content decreased as light intensity increased. We revealed that isovaleric acid is the main volatile component in V. fauriei, along with bornyl acetate and valeric acid.
Furthermore, changes in light intensity have been shown to influence volatile compounds levels, with optimal production of bornyl acetate and isovaleric acid occurring at 400L, whereas valeric acid production in maximized at 800L. It suggests that modifying ambient light conditions offers a viable approach for optimizing the production of desired compounds.
Overall, the findings indicate that a light intensity of 400L is the ideal in walk-in growth chamber, improving plant biomass, minimizing photoinhibition effect, and maximizing volatile compound production, compared to other light intensities.
To determine the optimal light cultivation conditions for maximizing volatile compound production in V. fauriei within a walk-in growth chamber, further study will focus on exploring individual light factors such as photoperiod, intensity, and quality. The goal is to identify the best combination of these factors to achieve a synergistic effect.
Acknowledgments
This work was supported by a Cooperative Research Program of Agriculture Science and Technology Development (PJ01667802) funded by the Rural Development Administration, Korea. In addition, this research was supported by Korea Basic Science Institute(National research Facilities and Equipment Center) grant funded by the Ministry of Education(Grant No. 2023R1A6C101B022).
References
-
Alvarenga ICA, Pacheco FV, Silva ST, Bertolucci SKV and Pinto JEBP. (2015). In vitro culture of Achillea millefolium L.: Quality and intensity of light on growth and production of volatiles. Plant Cell, Tissue and Organ Culture. 122:299-308.
[https://doi.org/10.1007/s11240-015-0766-7]

-
Bankole AE, Umebese CE, Ubajaka S and Asekun OT. (2022). Light intensity alters the growth and quality of volatile components from hexane extracts of Hyptis suaveolens(Family Lamiaceae): A medicinal plants. Asian Journal of Biological and Life Sciences. 11:811-818.
[https://doi.org/10.5530/ajbls.2022.11.108]

-
Bian ZH, Yang QC and Liu WK. (2015). Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. Journal of the Science of Food and Agriculture. 95:869-877.
[https://doi.org/10.1002/jsfa.6789]

-
Cavallaro V and Muleo R. (2022). The effect of LED light spectra and intensities on plant growth. Plants. 11:1911. https://www.mdpi.com/2223-7747/11/15/1911, (cited by 2024 Dec. 18).
[https://doi.org/10.3390/plants11151911]

-
Dyayiya NA, Oyemitan IA, Matewu R, Oyedeji OO, Oluwafemi SO, Nkeh-Chungag BN, Songca SP and Oyedeji AO. (2016). Chemical analysis and biological potential of Valerian root as used by herbal practitioners in the Eastern Cape Province, South Africa. African Journal of Traditional, Complementary and Alternative Medicines. 13:114-122.
[https://doi.org/10.21010/ajtcam.v13i1.16]

-
Feng L, Raza MA, Li Z, Chen Y, Khalid MHB, Du J, Liu W, Wu X, Song C, Yu L, Zhang Z, Yuan S, Yang W and Yang F. (2019). The influence of light intensity and leaf movement on photosynthesis characteristics and carbon balance of soybean. Frontiers in Plant Science. 9:1952. https://www.frontiersin.org/journals/plant-science/articles/10.3389/fpls.2018.01952/full, (cited by 2024 Oct. 30).
[https://doi.org/10.3389/fpls.2018.01952]

-
Gong WZ, Jiang CD, Wu YS, Chen HH, Liu WY and Yang WY. (2015). Tolerance vs. avoidance: Two strategies of soybean (Glycine max) seedlings in response to shade in intercropping. Photosynthetica. 53:259-268.
[https://doi.org/10.1007/s11099-015-0103-8]

-
Han R, Nusbaum O, Chen X and Zhu Y. (2020). Valeric acid suppresses liver cancer development by acting as a novel HDAC inhibitor. Molecular Therapy-Oncolytics. 19:8-18.
[https://doi.org/10.1016/j.omto.2020.08.017]

- Jin HS, Jin ML, Lee MY, Park HJ, Nam JH, Hwang B and Hwang SJ. (2007). Effect of elicitors on the production of valepotriates and valerenic acid in the adventitious roots of Valeriana fauriei var. dasycarpa Hara. Korean Journal of Medicinal Crop Science. 15:241-245.
-
Kruve A. (2020). Strategies for drawing quantitative conclusions from nontargeted liquid chromatography-high-resolution mass spectrometry analysis. Analytical Chemistry. 92:4691-4699.
[https://doi.org/10.1021/acs.analchem.9b03481]

-
Lazzarini LES, Bertolucci SKV, Pacheco FV, Santos JD, Silva ST, Carvalho AAD and Pinto JEBP. (2018). Quality and intensity of light affect Lippia gracilis Schauer plant growth and volatile compounds in vitro. Plant Cell, Tissue and Organ Culture. 135:367-379.
[https://doi.org/10.1007/s11240-018-1470-1]

-
Li J, Li X, Wang C, Zhang M, Ye M and Wang Q. (2022). The potential of Valeriana as a traditional Chinese medicine: traditional clinical applications, bioactivities, and phytochemistry. Frontiers in Pharmacology. 13:973138. https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2022.973138/full, (cited by 2024 Oct. 30).
[https://doi.org/10.3389/fphar.2022.973138]

-
Lichtenthaler HK and Buschmann C. (2001). Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Current Protocols in Food Analytical Chemistry. 1:F4.3.1-F4.3.8.
[https://doi.org/10.1002/0471142913.faf0403s01]

-
Patočka J and Jakl J. (2010). Biomedically relevant chemical constituents of Valeriana officinalis. Journal of Applied Biomedicine. 8:11-18.
[https://doi.org/10.2478/v10136-009-0002-z]

-
Powles SB. (1984). Photoinhibition of photosynthesis induced by visible light. Annual Review of Plant Physiology. 35:15-44.
[https://doi.org/10.1146/annurev.arplant.35.1.15]

-
Roeber VM, Bajaj I, Rohde M, Schmülling T and Cortleven A. (2021). Light acts as a stressor and influences abiotic and biotic stress responses in plants. Plant, Cell and Environment. 44:645-664.
[https://doi.org/10.1111/pce.13948]

-
Sato R, Ito H and Tanaka A. (2015). Chlorophyll b degradation by chlorophyll b reductase under high-light conditions. Photosynthesis Research. 126:249-259.
[https://doi.org/10.1007/s11120-015-0145-6]

-
Shafiq I, Hussain S, Raza MA, Iqbal N, Asghar MA, Raza A, Fan YF, Mumtaz M, Shoaib M, Ansar M, Manaf A, Yang WY and Yang F. (2021). Crop photosynthetic response to light quality and light intensity. Journal of Integrative Agriculture. 20:4-23.
[https://doi.org/10.1016/S2095-3119(20)63227-0]

-
Sharma A, Kumar V, Shahzad B, Ramakrishnan M, Sidhu GPS, Bali AS, Handa N, Kapoor D, Yadav P, Khanna K, Bakshi P, Rehman A, Kohli SK, Khan EA, Parihar RD, Yuan H, Thukral AK, Bhardwaj R and Zheng B. (2020). Photosynthetic response of plants under different abiotic stresses: A review. Journal of Plant Growth Regulation. 39:509-531.
[https://doi.org/10.1007/s00344-019-10018-x]

-
Silva ST, Bertolucci SKV, Cunha SHBD, Lazzarini LES, Tavares MC and Pinto JEBP. (2017). Effect of light and natural ventilation systems on the growth parameters and carvacrol content in the in vitro cultures of Plectranthus amboinicus (Lour.) Spreng. Plant Cell, Tissue and Organ Culture. 129:501-510.
[https://doi.org/10.1007/s11240-017-1195-6]

-
Thoma F, Somborn-Schulz A, Schlehuber D, Keuter V and Deerberg G. (2020). Effects of light on secondary metabolites in selected leafy greens: a review. Frontiers in Plant Science. 11:497. https://www.frontiersin.org/journals/plant-science/articles/10.3389/fpls.2020.00497/full, (cited by 2024 Oct. 30).
[https://doi.org/10.3389/fpls.2020.00497]

-
Wang J, Zhao J, Liu H, Zhou L, Liu Z, Wang J, Han J, Yu Z and Yang F. (2010). Chemical analysis and biological activity of the essential oils of two Valerianaceous species from China: Nardostachys chinensis and Valeriana officinalis. Molecules. 15:6411-6422. https://www.mdpi.com/1420-3049/15/9/6411, (cited by 2024 Oct. 30).
[https://doi.org/10.3390/molecules15096411]

-
Wang Z, Hwang SH, Kim JH and Lim SS. (2017). Anti-obesity effect of the above-ground part of Valeriana dageletiana Nakai ex F. Maek extract in high-fat diet-induced obese C57BL/6N mice. Nutrients. 9:689. https://www.mdpi.com/2072-6643/9/7/689, (cited by 2024 Oct 30).
[https://doi.org/10.3390/nu9070689]

-
Yang F, Fan Y, Wu X, Cheng Y, Liu Q, Feng L, Chen J, Wang Z, Wang X, Yong T, Liu W, Liu J, Du J, Shu K and Yang W. (2018). Auxin-to-gibberellin ratio as a signal for light intensity and quality in regulating soybean growth and matter partitioning. Frontiers in Plant Science. 9:56. https://www.frontiersin.org/journals/plant-science/articles/10.3389/fpls.2018.00056/full, (cited by 2024 Oct. 30).
[https://doi.org/10.3389/fpls.2018.00056]

-
Zhang M, Zhao D, Ma Z, Li X and Xiao Y. (2009). Growth and photosynthetic capability of Momordica grosvenori plantlets grown photoautotrophically in response to light intensity. HortScience. 44:757-763.
[https://doi.org/10.21273/HORTSCI.44.3.757]

-
Zhao ZJ, Sun YL and Ruan XF. (2023). Bornyl acetate: A promising agent in phytomedicine for inflammation and immune modulation. Phytomedicine. 114:154781. https://www.sciencedirect.com/science/article/pii/S0944711323001423, (cited by 2024 Oct. 30).
[https://doi.org/10.1016/j.phymed.2023.154781]
