
Effect of Sodium Chloride on Flavone Biosynthesis and Antioxidant Activitiesin Hairy Root Culture of Scutellaria baicalensis
This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Scutellaria baicalensis is a traditional medicinal plant that is rich in bioactive flavonoids, the accumulation of which can be impacted by environmental factors, including salinity. We aimed to determine the effects of different sodium chroride (NaCl) concentrations on the growth, flavonoid biosynthesis, and antioxidant activity of S. baicalensis hairy root cultures.
We examined the effects of NaCl at various concentrations (0, 30, 50, 100, 200, and 300 mM) on biomass production, flavonoid content, and antioxidant properties of S. baicalensis hairy roots and found that biomass production was impaired by salinity, with 50 mM NaCl significantly reducing the biomass. High performance liquid chromatography of flavonoids at different salinity levels indicated high levels of baicalin, baicalein, and wogonin at an NaCl concentration of 100 mM (moderate salinity-induced metabolic activation). Flavonoid synthesis was inhibited under high salinity conditions (200 mM - 300 mM NaCl); under these conditions, some flavonoids were undetectable. Higher radical scavenging and redox potentials were detected at an NaCl concentration of 100 mM, with lower IC50 values in DPPH and ABTS, and reducing power antioxidant assays (compared to control conditions).
These results emphasize the importance of moderate salt stress in promoting bioactive compound production by S. baicalensis, providing information on its potential for cultivation under stress-adapted conditions and practical applications for medical use.
Keywords:
Scutellaria baicalensis, Antioxidant, Flavonoid, High Performance Liquid Chromatography, NaCl TreatmentINTRODUCTION
Flavonoids play a vital role in environmental adaptations of plants, and are the most widely distributed secondary metabolites (Liu et al., 2021), with prominent applications in food and medicine.
Flavonoids have a C6-C3-C6 carbon skeleton structure characterized by at least two aromatic rings (labeled A and B) joined at the three carbons chain that has the potential to cyclize with ring A to form a heterocyclic ring containing oxygen ring C (Wang et al., 2018).
Flavonoids are further characterized as flavones, flavanols, flavanones, flavanols, isoflavones as well as leucoanthocyanidins and chalcones (Panche et al., 2016).
Flavones (from the Latin word flavus, “yellow”) are plant secondary metabolites found in plants and fungi and naturally exhibit yellow color and belong to one of the major groups of flavonoids that share a common 2-phenylchromen-4-one (2-phenyl-1-benzopyran-4-one) skeletal structure (Panche et al., 2016). Flavone is a prominent group of specialized metabolites that have essential functions during both plant signaling and defense strategy, however, many researchers find flavones are also valuable compounds in the human diet that can yield huge health-promoting benefits (Martens and Mithöfer, 2005; Jiang et al., 2016; Hostetler et al., 2017).
The root of S. baicalensis has been commonly used in Chinese herbal medicine and is rich in various bioactive flavones including major active compounds baicalin, baicalein, and wogonin (Kim et al., 2014a; Kim et al., 2014b; Liang et al., 2017).
Previous studies reported that wogonin exerts natural characteristics together with anti-cancer, restoring bonds in an inflammatory state, toward bacterial and viral infection (Huynh et al., 2020; Li et al., 2021; Wang et al., 2022).
Baicalein has conventionally been applied against a broad spectrum such as antioxidant, anti-inflammatory, anti-hepatotoxic, antiviral, and anticancer activities as well (Im et al., 2012; Sowndhararajan et al., 2017; Chagas et al., 2022; Chandrashekar and Pandi, 2022; Cui et al., 2022).
Soil salinity is another major abiotic stress that severely affects crop growth and yields around the world. That salinity induces osmotic pressure, ion toxicity, anabolic disorders, and oxidative stress (Munns and Tester, 2008; Arzani and Ashraf, 2016). Such factors may lead to physiological and biochemical abnormalities in plant tissues.
Low molecular weight non-enzymatic antioxidants are produced by plant cells and these phenolic compounds play a significant role in eliminating the ROS (Hodaei et al., 2018).
Due to their ability to protect biological systems from different kinds of oxidative stresses, phenolic compounds are important for redox homeostasis and could be a possible target in developing stress tolerance approaches in plants (Trchounian et al., 2016). So, the homeostasis of ROS and phytochemicals (such as polyphenols and flavonoids) also possibly influenced the plant adaptation to salt stress.
The objectives of this study were to determine the effect of salinity treatment with different concentrations of NaCl on flavone biosynthesis and their antioxidant activities in the hairy root culture of S. baicalensis.
MATERIALS AND METHODS
1. Establishment of hairy root culture
Hairy root cultures were developed from S. baicalensis as per the methods reported earlier by Park et al. (2021).
Every month, the hairy roots of S. baicalensis were transferred to a fresh agar-solidified SH (Schenk and Hildebrandt, 1972) medium. For the next experiment, the hairy roots are taken out from the agar medium and loaded into liquid SH culture.
Every 10 days portion of hairy root cultures is sub-cultured and preserved in SH liquid medium. The hairy root cultures were kept in a growth chamber at 25℃ with shaking at 100 rpm under standard cool white fluorescent light providing an illuminance of 35 mol․s-1․m-2 for a photoperiod of 16 h.
2. NaCl treatment in hairy root culture
SH liquid culture medium was supplemented with increasing NaCl concentrations (0, 30, 50, 100, 200, and 300 mM) to evaluate their effect on the growth and flavone production of S. baicalensis hairy root culture.
Harvested hairy roots were freeze-dried (to determine dry weight) at the end of 10-day cultures for further analysis. Each culture condition was conducted in triplicate.
3. Analysis of individual flavonoids using HPLC
HPLC equipment for S. baicalensis flavonoid analysis appeared as NS-4000 series (Futecs, Daejeon, Korea), which consisted of a pump, autosampler, column oven, and UV-vis detector.
The method from Lim et al. (2024). The column oven temperature (C18, 250 ㎜ × 4.6 ㎜, 5 ㎛, RStech, Daejeon, Korea) was set at 30℃ and solvent A consisted of distilled water containing 0.2% formic acid while solvent B consisted of methanol (99.9%). It was developed using a gradient at a flow rate of 1.0 ㎖/min. The injection volume was 60 ㎕, and the wavelength of the UV detector was set to 275 ㎚.
4. Quantification of total phenolic and flavonoid contents
The Folin-Ciocalteaus' phenol reagent reduction procedure was adopted in order to determine the total phenolic (TP) contents in S. baicalensis, following an earlier report from Lim et al. (2024), which used methanol-based extracts. The TP contents were determined at 760 ㎚ wavelength with a UV-vis spectrophotometer.
We estimated the total flavonoid (TF) contents in S. baicalensis, following a previous report from Lim et al. (2024). The absorbances were measured at a wavelength of 415 ㎚. The TP and TF contents were estimated using the calibration curves of gallic acid and quercetin, respectively.
5. In vitro antioxidant activities
We performed the widely used in vitro antioxidant assays, such as DPPH, ABTS, and reducing power assays. The experimental methods were followed as previously reported by Lim et al. (2024).
Ascorbic acid was used as a positive control at a concentration of 31.25 ㎎/ℓ. The IC50 values of DPPH and ABTS were computed using a plotted curve, following a previous report from Lim et al. (2024).
6. Statistical analysis
The results are the mean value and standard deviation (SD) of three replicates of all results. Statistical significance analysis was performed using SPSS 20 (SPSS Inc., Chicago, IL, USA) with Duncan’s Multiple Range Test (DMRT) at the 5% level (p < 0.05), and GraphPad Prism 8 (GraphPad Software, San Diego, USA) with Sidak's Multiple Comparison Test (*p < 0.05; **p < 0.01, and ***p < 0.001).
RESULTS
1. Phenotypic factors of different NaCl concentration treated S. baicalensis
The effect of different NaCl concentrations on the dry weight of S. baicalensis hairy roots highlights a gradual decline in biomass as salt concentration increases. At NaCl 0, 30, and 50 mM, the dry weight remains relatively high (–0.3 g/30 ㎖) and there is no statistical difference between the treatments.
This suggests that low to moderate salinity levels do not significantly impact root biomass production. However, at 100 mM NaCl, a marked decrease is observed, with dry weight significantly reduced.
At 200 and 300 mM NaCl, the dry weight declines further, reflecting no significant difference between them. This pattern demonstrates that high salinity levels adversely affect biomass accumulation, likely due to osmotic stress or ionic toxicity, suggesting a salt sensitivity threshold beyond 50 mM.
These findings provide insight into the tolerance limits of S. baicalensis roots under saline conditions, with potential implications for its cultivation in high-salinity environments (Fig. 1).
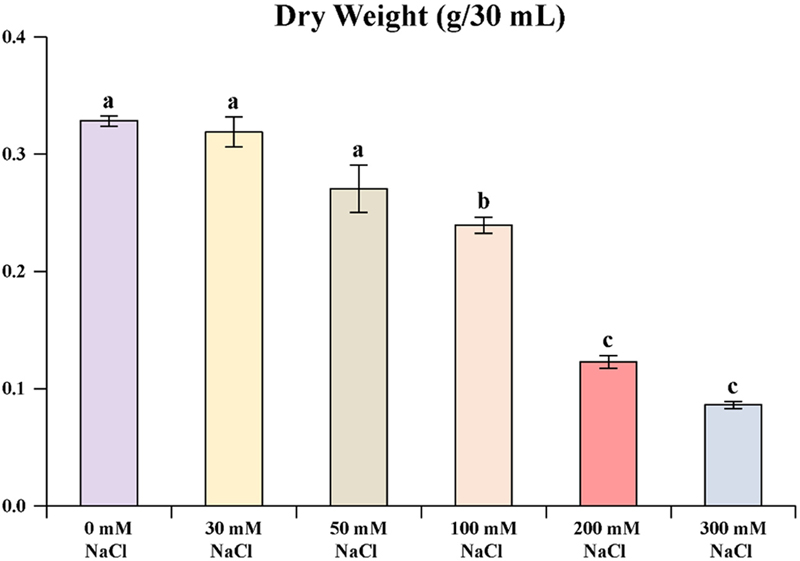
The dry weight of S. baicalensis hairy root culture was treated with different concentrations of NaCl.Values are means ± standard deviation of 3 replicates. nd; not detected. Means with each column followed by different alphabetical letters (a-c) indicating significant differences at 5% according to Duncan’s Multiple Range Test (DMRT, *p < 0.05).
2. Content of Baicalin, baicalein, and wogonin in different NaCl concentration treated S. baicalensis
Fig. 2. represents the impact of varying NaCl concentrations on three flavonoids in S. baicalensis: baicalin, baicalein, and wogonin. In all cases, the content of these flavonoids demonstrates a dynamic response to increasing salinity levels. Baicalin content peaks significantly at 100 mM NaCl (around 13 ㎎/g DW), with this concentration statistically distinct from all others.
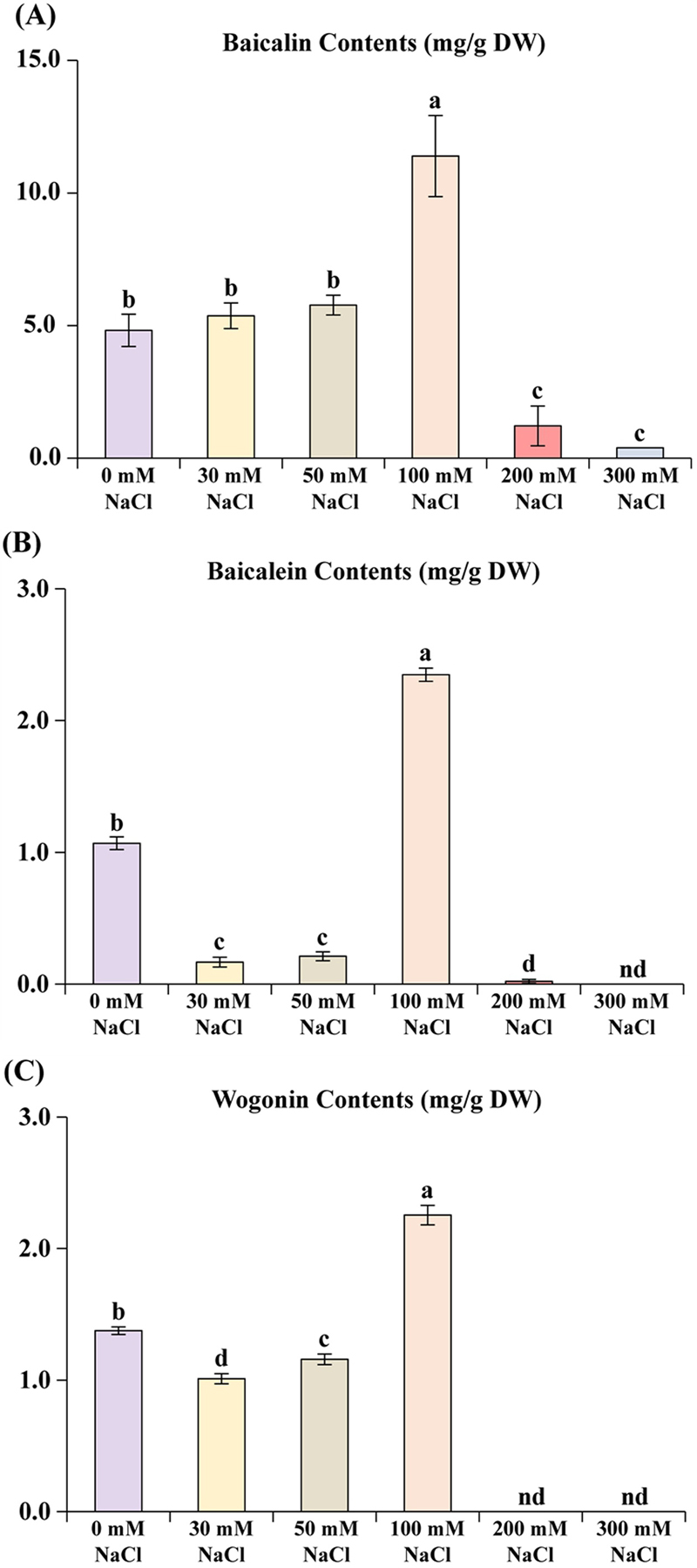
Flavonoid contents in S. baicalensis hairy root culture exposed to different NaCl concentrations.(A) Baicalin contents variation treated NaCl in S. baicalensis hairy roots, (B) baicalein contents variation treated NaCl in S. baicalensis hairy roots, and (C) wogonin contents variation treated NaCl in S. baicalensis hairy roots. Values means ± standard deviation of 3 replicates. nd: not detected. Means with each column followed by different alphabetical letters a-c indicating significant differences at at 5% according to Duncan’s Multiple Range Test (DMRT, *p < 0.05).
Lower concentrations (0, 30, and 50 mM) yield moderate baicalin levels (–5 ㎎/g DW), while higher concentrations (200 and 300 mM) show a sharp decline, suggesting potential damage to biosynthesis pathways under extreme salinity.
Similarly, baicalein content also peaks at 100 mM NaCl (–2.5 ㎎/g DW), showing that this concentration optimizes flavonoid accumulation, likely as a stress response mechanism. At 0 mM NaCl, moderate baicalein production occurs, followed by further reductions at 30 and 50 mM. Beyond 100 mM, levels drop sharply, with 200 mM yielding minimal amounts, and 300 mM showing undetectable content.
Wogonin follows a comparable trend, with a peak at 100 mM NaCl (–2.2 ㎎/g DW), while c ontent d eclines a s NaC l concentration increases from 0 to 50 mM. Notably, wogonin is undetectable at both 200 and 300 mM NaCl, highlighting its sensitivity to severe salinity stress (Fig. 2).
These findings suggest that 100 mM NaCl triggers a beneficial stress response, maximizing flavonoid production, whereas excessive salinity (200 mM - 300 mM) disrupts metabolic processes, diminishing flavonoid synthesis and accumulation. This trend emphasizes a critical threshold for optimal stress-induced flavonoid production in S. baicalensis.
3. Total phenol and total flavonoid content of different NaCl concentration treated S. baicalensis
TP content gradually increased with increasing concentration of NaCl. At the lowest concentrations (30 and 50 mM NaCl) and the highest concentrations (200 and 300 mM NaCl), the TP content was significantly lower than that of the control (0 mM NaCl). The highest TP content was achieved in the hairy root treated with 100 mM NaCl, whereas the lowest was obtained in the 300 mM NaCl treatment (Table 1). The TF content also showed a similar trend to that of the TP content. At the minimum NaCl concentration (30 and 50 mM NaCl) the TF content gradually increased, whereas at the highest NaCl concentrations (200 and 300 mM NaCl) the TF content was significantly decreased.

Total phenolic and total flavonoid content of S. baicalensis hairy root culture treated with different NaCl concentrations.
In both TP and TF content, the 100 mM NaCl showed significantly higher content than that of control and other NaCl-treated hairy root cultures. Hence, for the antioxidant analyses, we selected 100 mM NaCl as a treated culture, whereas 0 mM NaCl) were selected as a control .
4. Antioxidative activity of different NaCl concentration treated S. baicalensis
DPPH and ABTS radical scavenging activities of S. baicalensis extract under two NaCl treatment conditions (0 mM and 100 mM NaCl) across different extract concentrations (31.25 to 1,000 ㎎/ℓ).
Fig. 3a shows the DPPH radical scavenging activity, where ascorbic acid serves as a positive control with - 85% scavenging activity. The DPPH activity gradually increases with higher extract concentrations, with 100 mM NaCl-treated samples consistently showing higher scavenging activity than the 0 mM samples.
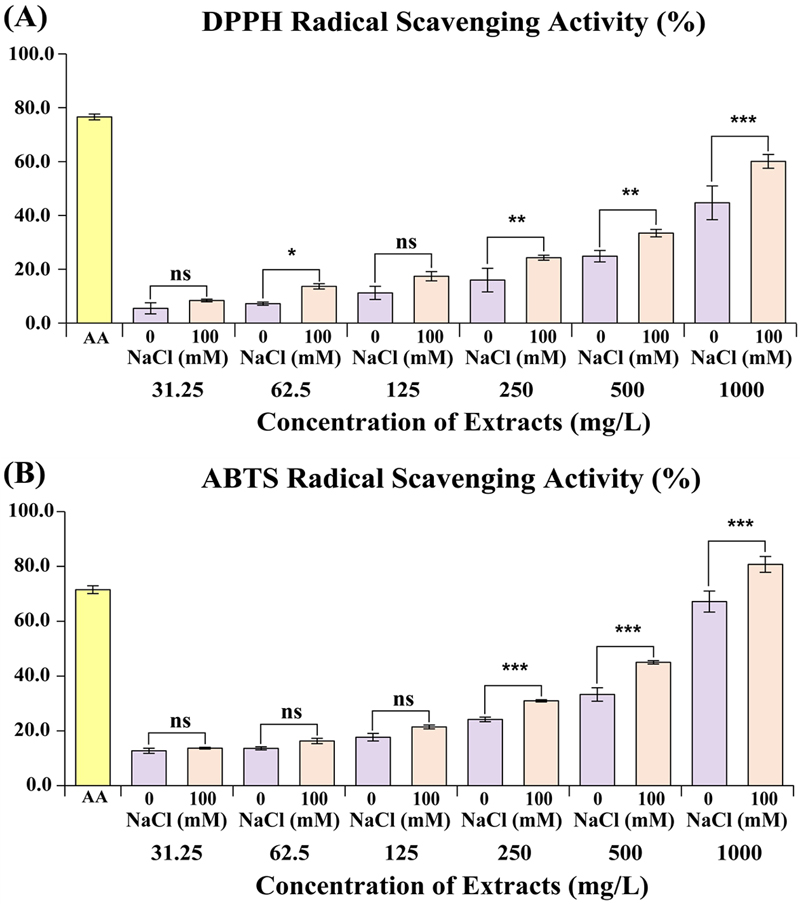
Antioxidant activities of S. baicalensis hairy root culture treated with 0 mM and 100 mM NaCl concentrations.(A) DPPH radical scavenging activity (%); (B) ABTS radical scavenging activity (%). Values are means ± standard deviation of 3 replicates. The x-axis represents the concentration of each extract, and the y-axis represents the sample’s radical scavenging activity. AA; ascorbic acid, ns; not significant. Asterisks indicate significant differences between the S. baicalensis hairy root culture treated with 0 mM and 100 mM NaCl concentrations, as determined by Sidak's multiple comparison test (*p < 0.05, **p < 0.01, and ***p < 0.001).
At 1,000 ㎎/ℓL, both NaCl treatments exhibit their highest activities, with 100 mM NaCl achieving around 80% scavenging, at lower concentrations (31.25 to 125 ㎎/ℓ), there is little to no significant difference, except for a slight difference at 62.5 ㎎/ℓ, reflecting a threshold concentration for noticeable antioxidant activity (Fig. 3).
Figure 3b displays the ABTS radical scavenging activity, with a similar pattern as the DPPH assay. Ascorbic acid shows 65% scavenging activity, and extract activity increases with concentration. Again, the 100 mM NaCl-treated samples exhibit significantly higher ABTS activity at higher concentrations (500 and 1,000 ㎎/ℓ).
At lower concentrations (31.25 ㎎/ℓ – 125 ㎎/ℓ), no significant differences are observed between NaCl treatments. These results suggest that salt stress enhances the antioxidant capacity of S. baicalensis, possibly due to an adaptive response that boosts the production of antioxidant metabolites. The difference in scavenging activity between treatments demonstrates the importance of stress conditions in modulating the plant's bioactive properties, with potential implications for the use of S. baicalensis extracts in health-related applications (Fig. 3).
The IC50 values (concentration required to reduce initial free radicals by 50%) for both DPPH and ABTS assays in S. baicalensis under two salinity treatments: 0 mM NaCl and 100 mM NaCl. Lower IC50 values indicate stronger antioxidant activity, as less extract is needed to achieve 50% radical scavenging. For DPPH, the 100 mM NaCl treated sample shows a significantly lower IC50 value (0.8 ± 0.01 ㎎/㎖) compared to the 0 mM NaCl sample (1.14 ± 0.02 ㎎/㎖), suggesting that salt stress enhances the antioxidant capacity for DPPH scavenging (Table 2).

IC50 value of DPPH and ABTS assays of S. baicalensis hairy root culture treated with 0 mM and 100 mM NaCl concentrations.
Similarly, for the ABTS assay, the 100 mM NaCl treatment also results in a lower IC50 value (0.55 ± 0.01 ㎎/㎖) compared to 0 mM NaCl (0.73 ± 0.0 ㎎/㎖). The improvement in antioxidant efficiency under 100 mM NaCl implies that salinity induces stress responses that boost the production of antioxidant metabolites, potentially increasing the plant’s ability to neutralize free radicals (Table 2).
However, there is no significant difference was found in the 100 mM NaCl, which indicates that the difference in antioxidant capacity for the ABTS assay is not statistically significant, suggesting variability in how different types of radicals respond to salt-induced changes in the plant. These findings highlight the complex relationship between environmental stress and bioactive compound production in S. baicalensis.
The reducing power of S. baicalensis extracts treated with different NaCl concentrations (0 mM and 100 mM) across a range of extract concentrations (31.25 to 1,000 ㎎/ℓ). Ascorbic acid, used as the positive control, demonstrates the highest reducing power (–0.3), indicating strong electron-donating ability. For the extracts, the reducing power increases proportionally with concentration.
At low concentrations (31.25 ㎎/ℓ), both NaCl treatments exhibit similar and minimal reducing power, with no statistically significant difference (ns). However, at 62.5 and 125 ㎎/ℓ, the 100 mM NaCl-treated samples show significantly higher reducing power, suggesting enhanced antioxidant potential under moderate salt stress. This trend becomes more pronounced at higher concentrations: at 250 ㎎/ℓ, the difference becomes highly significant.
The overall results suggest that salt stress (100 mM NaCl) enhances the reducing power of S. baicalensis extracts, likely due to an increase in antioxidant compounds that act as electron donors to neutralize free radicals (Fig. 4). This improvement in reducing power indicates that salinity stress promotes the production of bioactive compounds with enhanced redox potential, making 100 mM NaCl-treated extracts more effective in antioxidant assays. These findings highlight the role of environmental stress in influencing the antioxidant properties of S. baicalensis, with potential implications for its use in therapeutic and health-related applications.
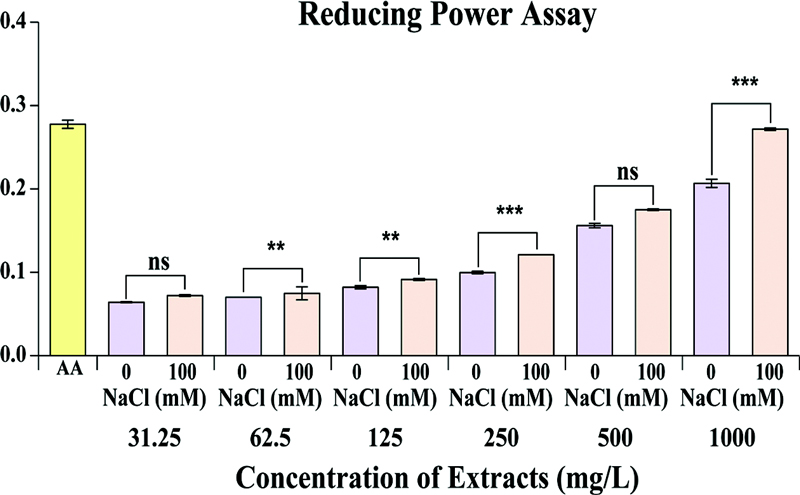
Reducing power assay of S. baicalensis hairy root culture treated with 0 mM and 100 mM NaCl concentrations.Values are means ± standard deviation of 3 replicates. The x-axis represents the concentration of each extract, and the y-axis represents the sample’s reducing power. AA; ascorbic acid, ns; not significant. Asterisks indicate significant differences between the S. baicalensis hairy root culture treated with 0 mM and 100 mM NaCl concentrations, as determined by Sidak's multiple comparison test (*p < 0.05; **p < 0.01, and ***p < 0.001).
The effect of varying concentrations of NaCl on biomass production. As the NaCl concentration increases, there is a clear decline in biomass, suggesting that higher salinity levels negatively a ffect growth. At 0 mM NaC l, the b iomass is a t its highest, with a mean value of 0.33 ± 0.01 g/30 mL. However, as the concentration rises to 30 mM and 50 mM, the biomass decreases to 0.32 ± 0.02 g/30㎖ and 0.27 ± 0.03 g/30 ㎖, respectively. This trend continues more sharply at higher concentrations, with biomass values reducing to 0.24 ± 0.01 g/30㎖ at 100 mM, 0.12 ± 0.01 g/30㎖ at 200 mM, and reaching the lowest value of 0.09 ± 0.01 g/30㎖ at 300 mM NaCl.
These findings indicate a dose-dependent inhibition of biomass production with increasing salinity, likely due to the osmotic stress and ionic toxicity imposed by elevated NaCl concentrations (Table 2).
The impact of different NaCl concentrations on the flavonoid content, specifically focusing on baicalin, baicalein, and wogonin, measured in ㎎/g dry weight (DW). Baicalin content initially increases with mild salinity, reaching a peak of 11.39 ± 1.53 ㎎/g DW at 100 mM NaCl, indicating that moderate salt stress may enhance its accumulation.
However, at higher concentrations (200 mM and 300 mM), baicalin levels sharply decline to 1.22 ± 0.75 ㎎/g DW and 0.39 ± 0 ㎎/g DW, respectively, suggesting a detrimental effect of excessive salinity. Similarly, baicalein content peaks at 2.35 ± 0.05 ㎎/g DW at 100 mM NaCl but is drastically reduced at higher concentrations, with no detectable levels at 300 mM. Wogonin follows a comparable pattern, with a peak of 2.26 ± 0.07 ㎎/g DW at 100 mM, followed by a complete absence at 200 mM and 300 mM NaCl.
These results indicate that moderate salinity may stimulate flavonoid production, while excessive NaCl concentrations inhibit their accumulation, likely due to stress-induced metabolic limitations.
The antioxidant activity was measured through DPPH, ABTS, and reducing power assays, at varying concentrations of extracts under two NaCl treatments: 0 mM and 100 mM. In the DPPH assay, antioxidant activity improves with increasing extract concentration, with NaCl 100 mM consistently showing higher activity across all concentrations, reaching 60.08 ± 2.56 ㎎/ℓ at 1,000 ㎎/ℓ compared to 44.69 ± 6.27 ㎎/ℓ for NaCl 0 mM.
A similar trend is observed in the ABTS assay, where NaCl 100 mM treatment enhances antioxidant potential, peaking at 80.74 ± 2.89 ㎎/ℓ at 1,000 ㎎/ℓ, in contrast to 67.18 ± 3.83 ㎎/ℓ for NaCl 0 mM.
The reducing power assay also shows a slight improvement with NaCl 100 mM, where the activity increases from 0.07 ± 0.0 to 0.27 ± 0.0 at the highest concentration, compared to 0.21 ± 0.0 for NaCl 0 mM.
These results suggest that exposure to moderate salinity (100 mM NaCl) enhances the antioxidant properties of the extracts, likely due to an upregulation of stress-responsive metabolites, improving free radical scavenging and reducing power capabilities across the assays.
DISCUSSION
Salinity stress had an observable effect on the biomass of hairy roots, as shown by the decrease in root biomass with increasing NaCl concentration. At the early concentrations (0, 30, and 50 mM), biomass was only slightly diminished (0.3 g/30 ㎖) indicating that panicoids can tolerate low levels of salinity without serious growth impairment (Wang et al., 2018).
At 100 mM NaCl, biomass was significantly lower than in control conditions suggesting that the process of growth suppression associated with stress has begun. The drastic reduction in plant height at 200 and 300 mM NaCl reflects the high sensitivity of L. sativa germination to elevated salinity, presumably due to osmotic stress and ionic toxicity which hinder water uptake and disturb cellular homeostasis (Fang et al., 2023; Solov’eva et al., 2023).
These results correspond with the studies on other medicinal plants (e.g. S. baicalensis and S. pycnoclada) which reported that the high salinity reduced root biomass and inhibited metabolic activities in roots (Lee et al., 2013).
The S. baicalensis hairy roots showed a non-linear responsiveness in flavonoid biosynthesis to concentration of NaCl. The yield peak of baicalin, baicalein, and wogonin was at 100 mM NaCl treatment, indicating that medium salinity could activate a positive stress physiology that promotes the accumulation of flavonoids. At 100 mM NaCl, the content of baicalin increased to 11.39 ± 1.53 ㎎/g DW, and then dropped sharply at 200 mM and further declined at 300 mM NaCl suggesting that high salinity induces disturbances in metabolic pathways critical for flavonoids (Kim et al., 2012).
This trend corroborates with findings showing that stress conditions like salinity can enhance the production of secondary metabolites through cellular metabolic changes (Wang et al., 2018), but too high an extent of stress inhibits biosynthetic processes.
The significantly more potent antioxidant capacity of S. baicalensis extracts subjected to salt stress is confirmed by DPPH and ABTS assays.
DPPH scavenging activity at all extract concentrations showed higher antioxidant activity in the NaCl-treated sample (100 mM) than in the untreated samples, where 80% was DPPH scavenging activity at a concentration of extract 1,000 ㎎/ℓ.
In ABTS this pattern is similarly observed, with the NaCl 100 mM samples leading to an abolition of scavenging activity (80.74 ㎎/ℓ), significantly higher than for NaCl 0 mM. This indicates that NaCl stress activates a response, which causes the plant to be primed to synthesize antioxidant metabolites useful in defending against oxidative stress (Schenk and Hildebrandt, 1972). Nevertheless, the IC50 values indicated decreased antioxidant activity at 300 mM NaCl, which is an indication that high salinity stress can harm metabolic efficiency (Kim et al., 2012; Park et al., 2011).
The reducing power assay further demonstrated that the electron-donating ability of S. baicalensis extracts improves under moderate salt stress. At higher extract concentrations (250 ㎎/ℓ - 1,000 ㎎/ℓ), NaCl-treated samples (100 mM) showed significantly higher reducing power compared to untreated samples, suggesting enhanced production of bioactive compounds with redox potential. This increase in reducing power corresponds to the plant’s ability to synthesize flavonoids such as baicalin, baicalein, and wogonin, which possess strong antioxidant properties.
The observed trend is consistent with findings that salinity induces the accumulation of stress-responsive metabolites in hairy root cultures, enhancing their pharmacological properties (Hostetler et al., 2017; Park et al., 2021).
Studies on other strains of S. baicalensis and related species highlight similar trends in response to environmental stress. For example, S. pycnoclada showed higher flavonoid accumulation under optimized stress conditions, although the species lacked some biosynthetic enzymes present in S. baicalensis (Park et al., 2011; Solov’eva et al., 2023). This suggests that environmental factors play a crucial role in modulating secondary metabolism in these medicinal plants. Additionally, genetic modifications through metabolic engineering have been proposed as a strategy to further enhance flavonoid production under stress conditions (Park et al., 2021).
The findings offer vital information on the interaction of salinity conditions and metabolic behaviors in S. baicalensis. Flavonoid synthesizing and antioxidant activity are maximized by moderate salt stress (100 mM NaCl) revealing a crucial limit for adaptive responses to stress Hey, but high salinity (200 mM - 300 mM) hinders these processes and highlights the need for environmental management during cultivation.
Our results have valuable implications for the pharmaceutical industry because metabolic changes induced by stress on S. baicalensis may be further exploited to promote its therapeutic potential. Future studies should investigate more genetic and environmental interactions to maximize flavonoid production of this medicinal plant and develop sustainable cultivation approaches (Liu et al., 2021).
In conclusion, S. baicalensis responds dynamically to varying NaCl concentrations, with 100 mM NaCl enhancing flavonoid production (baicalin, baicalein, wogonin) and antioxidant activity through improved radical scavenging and reducing power. However, higher salinity (200–300 mM) impairs biomass and flavonoid synthesis due to osmotic stress and ionic toxicity. Moderate salinity thus offers a strategic approach to boost the plant’s pharmacological potential, while excessive levels hinder its benefits, highlighting the need for optimized growing conditions.
Acknowledgments
This research was supported by the Bio and Medical Tehcnology Development Program of the National Reserach Foundation(NRF) and funded by the Korean Government (MSIT, No. 2022M3E5E6018649).
References
-
Arzani A and Ashraf M. (2016). Smart engineering of genetic resources for enhanced salinity tolerance in crop plants. Critical Reviews in Plant Sciences. 35:146-189.
[https://doi.org/10.1080/07352689.2016.1245056]

-
Chagas MdSS, Behrens MD, Moragas-Tellis CJ, Penedo GX, Silva AR and Gonçalves-de-Albuquerque CF. (2022). Flavonols and flavones as potential anti‐inflammatory, antioxidant, and antibacterial compounds. Oxidative Medicine and Cellular Longevity. 2022:9966750. https://onlinelibrary.wiley.com/doi/full/10.1155/2022/9966750, (cited by 2024 Oct. 04).
[https://doi.org/10.1155/2022/9966750]

-
Chandrashekar N and Pandi A. (2022). Baicalein: A review on its anti‐cancer effects and mechanisms in lung carcinoma. Journal of Food Biochemistry. 46:e14230. https://onlinelibrary.wiley.com/doi/full/10.1111/jfbc.14230, (cited by 2024 Sep. 04).
[https://doi.org/10.1111/jfbc.14230]

-
Cui L, Yuan T, Zeng Z, Liu D, Liu C, Guo J and Chen Y. (2022). Mechanistic and therapeutic perspectives of baicalin and baicalein on pulmonary hypertension: A comprehensive review. Biomedicine and Pharmacotherapy. 151:113191. https://www.sciencedirect.com/science/article/pii/S0753332222005807, (cited by 2024 Sep. 09).
[https://doi.org/10.1016/j.biopha.2022.113191]

-
Fang Y, Liu J, Zheng M, Zhu S, Pei T, Cui M, Chang L, Xiao H, Yang J and Martin C. (2023). SbMYB3 transcription factor promotes root-specific flavone biosynthesis in Scutellaria baicalensis. Horticulture Research. 10:uhac266. https://academic.oup.com/hr/article/10/2/uhac266/6865346?login=false, (cited by 2024 Oct. 09).
[https://doi.org/10.1093/hr/uhac266]

-
Hodaei M, Rahimmalek M, Arzani A and Talebi M. (2018). The effect of water stress on phytochemical accumulation, bioactive compounds and expression of key genes involved in flavonoid biosynthesis in Chrysanthemum morifolium L. Industrial Crops and Products. 120:295-304.
[https://doi.org/10.1016/j.indcrop.2018.04.073]

-
Hostetler GL, Ralston RA and Schwartz SJ. (2017). Flavones: Food sources, bioavailability, metabolism, and bioactivity. Advances in Nutrition. 8:423-435.
[https://doi.org/10.3945/an.116.012948]

-
Huynh DL, Ngau TH, Nguyen NH, Tran G-B and Nguyen CT. (2020). Potential therapeutic and pharmacological effects of Wogonin: an updated review. Molecular Biology Reports. 47:9779-9789.
[https://doi.org/10.1007/s11033-020-05972-9]

-
Im AR, Kim YH, Uddin MR, Lee HW, Chae S.W, Kim YH, Jung WS, Kang BJ, Mun CS and Lee MY. (2012). Scutellaria baicalensis extracts and flavonoids protect rat L6 cells from antimycin a‐induced mitochondrial dysfunction. Evidence‐Based Complementary and Alternative Medicine. 2012:517965. https://onlinelibrary.wiley.com/doi/full/10.1155/2012/517965, (cited by 2024 Sep. 22).
[https://doi.org/10.1155/2012/517965]

-
Jiang N, Doseff AI and Grotewold E. (2016). Flavones: From biosynthesis to health benefits. Plants. 5:27. https://www.mdpi.com/2223-7747/5/2/27, (cited by 2024 Oct. 24).
[https://doi.org/10.3390/plants5020027]

-
Kim JK, Kim YS, Kim Y, Uddin MR, Kim YB, Kim HH, Park SY, Lee MY, Chung SO and Park SU. (2014a). Comparative analysis of flavonoids and polar metabolites from hairy roots of Scutellaria baicalensis and Scutellaria lateriflora. World Journal of Microbiology and Biotechnology. 30:887-892.
[https://doi.org/10.1007/s11274-013-1498-7]

-
Kim YB, Uddin MR, Kim Y, Park CG and Park SU. (2014b). Molecular cloning and characterization of tyrosine aminotransferase and hydroxyphenylpyruvate reductase, and rosmarinic acid accumulation in Scutellaria baicalensis. Natural Product Communications. 9:1311-1314.
[https://doi.org/10.1177/1934578X1400900923]

- Kim YS, Li X, Park WT, Uddin MR, Park NI, Kim YB, Lee MY and Park SU. (2012). Influence of media and auxins on growth and falvone production in hairy root cultures of baikal skullcap,'Scutellaria baicalensis'. Plant Omics. 5:24-27.
-
Lee SW, Kim YS, Uddin MR, Kwon DY, Kim YB, Lee MY, Kim SJ and Park SU. (2013). Resveratrol production from hairy root cultures of Scutellaria baicalensis. Natural Product Communications. 8:609-611.
[https://doi.org/10.1177/1934578X1300800517]

-
Li K, Liang Y, Cheng A, Wang Q, Li Y, Wei H, Zhou C and Wan X. (2021). Antiviral properties of baicalin: A concise review. Revista Brasileira de Farmacognosia. 31:408-419.
[https://doi.org/10.1007/s43450-021-00182-1]

-
Liang W, Huang X and Chen W. (2017). The effects of baicalin and baicalein on cerebral ischemia: A review. Aging and disease. 8:850. https://pmc.ncbi.nlm.nih.gov/articles/PMC5758355/, (cited by 2024 Nov. 02).
[https://doi.org/10.14336/AD.2017.0829]

-
Lim J, Kim K, Kwon DY, Kim JK, Sathasivam R and Park SU. (2024). Effects of different solvents on the extraction of phenolic and flavonoid compounds, and antioxidant activities, in Scutellaria baicalensis hairy roots. Horticulturae. 10:160. https://www.mdpi.com/2311-7524/10/2/160, (cited by 2024 Nov. 02).
[https://doi.org/10.3390/horticulturae10020160]

-
Liu W, Feng Y, Yu S, Fan Z, Li X, Li J and Yin H. (2021). The flavonoid biosynthesis network in plants. International Journal of Molecular Sciences. 22:12824. https://www.mdpi.com/1422-0067/22/23/12824, (cited by 2024 Oct. 06).
[https://doi.org/10.3390/ijms222312824]

-
Martens S and Mithöfer A. (2005). Flavones and flavone synthases. Phytochemistry. 66:2399-2407.
[https://doi.org/10.1016/j.phytochem.2005.07.013]

-
Munns R and Tester M. (2008). Mechanisms of salinity tolerance. Annual Review of Plant Biology. 59:651-681.
[https://doi.org/10.1146/annurev.arplant.59.032607.092911]

- Panche AN, Diwan AD and Chandra SR. (2016). Flavonoids: An overview. Journal of Nutritional Science. 5:e47. https://www.cambridge.org/core/journals/journal-of-nutritional-science/article/flavonoids-an-overview/C0E91D3851345CEF4746B10406908F52, (cited by 2024 Sep. 02).
-
Park CH, Xu H, Yeo HJ, Park YE, Hwang G-S, Park NI and Park SU. (2021). Enhancement of the flavone contents of Scutellaria baicalensis hairy roots via metabolic engineering using maize Lc and Arabidopsis PAP1 transcription factors. Metabolic Engineering. 64:64-73.
[https://doi.org/10.1016/j.ymben.2021.01.003]

- Park WT, Kim YS, Park NI, Kim HH, Lee SY and Park SU. (2011). Influence of different strains of Agrobacterium rhizogenes on hairy root induction and growth in Scutellaria baicalensis. Korean Journal of Agricultural Science. 38:213-217.
-
Schenk RU and Hildebrandt A. (1972). Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Canadian Journal of Botany. 50:199-204.
[https://doi.org/10.1139/b72-026]

-
Solov’eva AI, Stepanova AY, Panov YM and Gladkov EA. (2023). Metabolic characteristics of hairy root clones of Scutellaria pycnoclada and Scutellaria baicalensis. Processes. 11:2102. https://www.mdpi.com/2227-9717/11/7/2102, (cited by 2024 Nov. 02).
[https://doi.org/10.3390/pr11072102]

-
Sowndhararajan K, Deepa P, Kim M, Park SJ and Kim S. (2017). Baicalein as a potent neuroprotective agent: A review. Biomedicine and Pharmacotherapy. 95:1021-1032.
[https://doi.org/10.1016/j.biopha.2017.08.135]

-
Trchounian A, Petrosyan M, Sahakyan N. (2016). Plant cell redox homeostasis and reactive oxygen species. In Gupta DK. et al. (eds.). Redox state as a central regulator of plant cell stress responses. Springer International Publishing. Cham, Switzerland. p.25-50.
[https://doi.org/10.1007/978-3-319-44081-1_2]

-
Wang T-Y, Li Q and Bi K-S. (2018). Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian Journal of Pharmaceutical Sciences. 13:12-23.
[https://doi.org/10.1016/j.ajps.2017.08.004]

-
Wang X, Xie L, Long J, Liu K, Lu J, Liang Y, Cao Y, Dai X and Li X. (2022). Therapeutic effect of baicalin on inflammatory bowel disease: A review. Journal of Ethnopharmacology. 283: 114749. https://www.sciencedirect.com/science/article/abs/pii/S0378874121009788, (cited by 2024 Oct. 06).
[https://doi.org/10.1016/j.jep.2021.114749]
